Drug Information
Drug (ID: DG00920) and It's Reported Resistant Information
| Name |
Lovastatin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Lovastatin; 75330-75-5; mevinolin; Mevacor; MK-803; Altoprev; Lovalord; Nergadan; Artein; Monacolin K; 6alpha-Methylcompactin; Lovalip; Lovastatine [French]; Lovastatinum [Latin]; Lovastatina [Spanish]; 6-alpha-Methylcompactin; Mevinacor; Altocor; Mevlor; Sivlor; Hipovastin; Lovasterol; Cholestra; Closterol; Colevix; Hipolip; Lestatin; Lipivas; Lipofren; Lovastin; Lozutin; Paschol; Rodatin; Rovacor; Tecnolip; Teroltrat; Belvas; Lipdip; Taucor; UNII-9LHU78OQFD; MLS000069585; MSD 803; 9LHU78OQFD; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate; MK 803; 2beta,6alpha-Dimethyl-8alpha-(2-methyl-1-oxobutoxy)-mevinic acid lactone; SMR000058779; (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate; (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate; Lovastatine; CHEBI:40303; L-154803; MFCD00072164; Lovastatin (Mevacor); NSC-758662; (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-(2-(2R,4R)-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl (S)-2-methyl-butyrate; HSDB 6534; NCGC00023509-03; Lovastatina; Lovastatinum; C24H36O5; DSSTox_CID_784; DSSTox_RID_75788; DSSTox_GSID_20784; butanoic acid, 2-methyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester, (2S)-; Liposcler; 6 alpha-Methylcompactin; Rextat; Monakolin K; (1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (S)-2-methylbutanoate; (S)-(1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2-methylbutanoate; Mevacor (TN); 6.alpha.-Methylcompactin; CHEMBL503; Lovastatin & Primycin; Lovastatin (USP/INN); SR-05000001880; (1S-(1alpha(R*),3alpha,7beta,8beta(2S*,4S*),8abeta))-2-Methylbutanoic acid 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester; (S)-2-Methylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8aR)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one; 1,2,6,7,8,8a-Hexahydro-beta,delta-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobutyoxy)-1-naphthaleneheptanoic acid delta-lactone; BRN 3631989; Mevinolin from Aspergillus sp.; CCRIS 8092; 1cqp; Lovastatin,(S); ML-530B; Lovastatin [USAN:USP:INN:BAN]; (+)-mevinolin; (S)-((1S,3R,7S,8S,8aR)-8-(2-((2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl)-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl) 2-methylbutanoate; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate; Lovastatin- Bio-X; Prestwick_819; CAS-75330-75-5; Lovastatin [USAN]; Mevinolin (lovastatin); Lovastatin (Mevinolin); Opera_ID_1578; Prestwick0_000516; Prestwick1_000516; Prestwick2_000516; Prestwick3_000516; Spectrum3_001873; Spectrum5_001294; Lovastatin (MK-803); EC 616-212-7; SCHEMBL3136; US9115116, lovastatin; BIDD:PXR0113; BSPBio_000471; BSPBio_001265; BSPBio_003346; Butanoic acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (1S-(1alpha(R*),3alpha,7beta,8beta(2S*,4S*),8abeta))-; cid_53232; MLS001055358; MLS006011867; US9353061, Lovastatina; BIDD:GT0749; DivK1c_001032; SPECTRUM1503977; SPBio_002392; BPBio1_000519; GTPL2739; MEGxm0_000398; DTXSID5020784; SCHEMBL14227102; ACon0_000534; ACon1_000390; BDBM34168; HMS503O05; KBio1_001032; KBio3_002848; AOB5269; C10AA02; Simvastatin impurity, lovastatin-; NINDS_001032; HMS1569H13; HMS1792O07; HMS1923O13; HMS1990O07; HMS2089M06; HMS2093O03; HMS2096H13; HMS2236F07; HMS3039N16; HMS3259F10; HMS3268C03; HMS3403O07; HMS3412H19; HMS3676H19; HMS3713H13; HMS3884B03; Pharmakon1600-01503977; 2-Methyl-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester butanoic acid; ACT02620; ALBB-027272; Butanoic acid, 2-methyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-((2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (2S)-; HY-N0504; MK-803; LOVALIP; MEVACOR; ZINC3812841; Tox21_110888; Tox21_201475; Tox21_300268; BBL024473; CCG-39627; NSC633781; NSC758662; NSC779704; s2061; STK801953; AKOS005267139; Tox21_110888_1; CS-1990; DB00227; KS-1082; MCULE-4740518260; MCULE-7087866108; Mevinolin from Aspergillus sp., powder; NC00713; NSC 758662; NSC-633781; NSC-779704; IDI1_001032; NCGC00023509-04; NCGC00023509-05; NCGC00023509-06; NCGC00023509-07; NCGC00023509-08; NCGC00023509-09; NCGC00023509-10; NCGC00023509-11; NCGC00023509-13; NCGC00023509-14; NCGC00023509-16; NCGC00254157-01; NCGC00259026-01; 74133-25-8; AC-13961; BL164644; Butanoic acid, 2-methyl-, (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (2S)-; G226; SMR000673570; SBI-0051881.P002; L0214; N1632; EN300-52515; C07074; D00359; J10136; AB00052400-17; AB00052400_18; AB00052400_19; Mevinolin from Aspergillus sp., >=98% (HPLC); 330L755; A838030; A838383; Q417740; SR-01000000123; SR-01000000123-3; SR-05000001880-1; SR-05000001880-2; BRD-K09416995-001-06-8; BRD-K09416995-001-21-7; Z1258578375; (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-Hexahydro-8-(2-((4R,6R)-4-hydroxy-2-oxo-2H-pyran-6-yl)ethyl)-3,7-dimethylnaphtyl(S)-2-methylbutyrat; (2S)-(1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-Hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl-2-methyl butanoate; (2S)-2-Methylbutanoic acid (1S,3R,7S,8S,8aR)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2R,4R)-tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl]ethyl]-1-naphthalenyl ester; (2S)-2-methylbutanoic acid [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxo-2-oxanyl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] ester; (S)-((1S,3R,7S,8S,8AR)-8-(2-((2R,4R)-4-HYDROXY-6-OXO-TETRAHYDRO-2H-PYRAN-2-YL)ETHYL)-3,7-DIMETHYL-1,2,3,7,8,8A-HEXAHYDRONAPHTHALEN-1-YL) 2-METHYLBUTANOATE; (S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-((3R,5R)-4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl ester; [(1S,3R,7S,8S,8aR)-3,7-dimethyl-8-[2-[(2R,4R)-4-oxidanyl-6-oxidanylidene-oxan-2-yl]ethyl]-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] (2S)-2-methylbutanoate; [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-Hydroxy-6-oxo-tetrahydropyran-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2-methylbutanoate; 1S,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate; 8-[2-(4-Hydroxy-6-oxotetrahydro-2H-pyran-2-yl)ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydro-1-naphthale; Butanoic acid, 2-methyl-, 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-(2-(tetrahydro-4-hydroxy-6-- oxo-2H-pyran-2-yl)ethyl)-1-naphthalenyl ester, (1S-(1alpha(R*),3alpha,7beta,8beta(2S*,4S*),8abeta))-
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
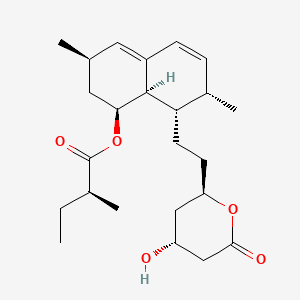
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[2]
|
||||
| Target | HMG-CoA reductase (HMGCR) | HMDH_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C24H36O5
|
||||
| IsoSMILES |
CC[C@H](C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C
|
||||
| InChI |
1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
|
||||
| InChIKey |
PCZOHLXUXFIOCF-BXMDZJJMSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Transcription factor PDR1 (PDR1) | [1] | |||
| Sensitive Disease | Fungal infection [ICD-11: 1F29-1F2F] | |||
| Molecule Alteration | Deletion mutation | Deleteion |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Saccharomyces cerevisiae strain Y12409 | 4932 | ||
| Saccharomyces cerevisiae strain Y13029 | 4932 | |||
| Saccharomyces cerevisiae strain Y13951 | 4932 | |||
| Saccharomyces cerevisiae strain Y14381 | 4932 | |||
| Sarcoma tissue | N.A. | |||
| Experiment for Molecule Alteration |
PCR | |||
| Experiment for Drug Resistance |
Spot Test | |||
| Mechanism Description | We investigated the susceptibility to lovastatin of S. cerevisiae strains deleted for PDR genes, responsible for exporting hydrophobic and amphiphilic drugs, such as lovastatin. Strains deleted for the genes tested, PDR1, PDR3, PDR5 and SNQ2, exhibited remarkably different phenotypes, with deletion of PDR5 causing the highest sensitivity to lovastatin. The study helped clarifying which pdr mutants to use in studies of physiological actions of statins in yeast. | |||
| Key Molecule: Pleiotropic ABC efflux transporter of multiple drugs (PDR5) | [1] | |||
| Sensitive Disease | Fungal infection [ICD-11: 1F29-1F2F] | |||
| Molecule Alteration | Deletion mutation | Deleteion |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Saccharomyces cerevisiae strain Y12409 | 4932 | ||
| Saccharomyces cerevisiae strain Y13029 | 4932 | |||
| Saccharomyces cerevisiae strain Y13951 | 4932 | |||
| Saccharomyces cerevisiae strain Y14381 | 4932 | |||
| Sarcoma tissue | N.A. | |||
| Experiment for Molecule Alteration |
PCR | |||
| Experiment for Drug Resistance |
Spot Test | |||
| Mechanism Description | We investigated the susceptibility to lovastatin of S. cerevisiae strains deleted for PDR genes, responsible for exporting hydrophobic and amphiphilic drugs, such as lovastatin. Strains deleted for the genes tested, PDR1, PDR3, PDR5 and SNQ2, exhibited remarkably different phenotypes, with deletion of PDR5 causing the highest sensitivity to lovastatin. The study helped clarifying which pdr mutants to use in studies of physiological actions of statins in yeast. | |||
|
|
||||
| Key Molecule: Transcription factor PDR1 (PDR1) | [1] | |||
| Sensitive Disease | Fungal infection [ICD-11: 1F29-1F2F] | |||
| Molecule Alteration | Deletion mutation | Deleteion |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Saccharomyces cerevisiae strain Y12409 | 4932 | ||
| Saccharomyces cerevisiae strain Y13029 | 4932 | |||
| Saccharomyces cerevisiae strain Y13951 | 4932 | |||
| Saccharomyces cerevisiae strain Y14381 | 4932 | |||
| Sarcoma tissue | N.A. | |||
| Experiment for Molecule Alteration |
PCR | |||
| Experiment for Drug Resistance |
Spot Test | |||
| Mechanism Description | We investigated the susceptibility to lovastatin of S. cerevisiae strains deleted for PDR genes, responsible for exporting hydrophobic and amphiphilic drugs, such as lovastatin. Strains deleted for the genes tested, PDR1, PDR3, PDR5 and SNQ2, exhibited remarkably different phenotypes, with deletion of PDR5 causing the highest sensitivity to lovastatin. The study helped clarifying which pdr mutants to use in studies of physiological actions of statins in yeast. | |||
| Key Molecule: Transcription factor PDR3 (PDR3) | [1] | |||
| Sensitive Disease | Fungal infection [ICD-11: 1F29-1F2F] | |||
| Molecule Alteration | Deletion mutation | Deleteion |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Saccharomyces cerevisiae strain Y12409 | 4932 | ||
| Saccharomyces cerevisiae strain Y13029 | 4932 | |||
| Saccharomyces cerevisiae strain Y13951 | 4932 | |||
| Saccharomyces cerevisiae strain Y14381 | 4932 | |||
| Sarcoma tissue | N.A. | |||
| Experiment for Molecule Alteration |
PCR | |||
| Experiment for Drug Resistance |
Spot Test | |||
| Mechanism Description | We investigated the susceptibility to lovastatin of S. cerevisiae strains deleted for PDR genes, responsible for exporting hydrophobic and amphiphilic drugs, such as lovastatin. Strains deleted for the genes tested, PDR1, PDR3, PDR5 and SNQ2, exhibited remarkably different phenotypes, with deletion of PDR5 causing the highest sensitivity to lovastatin. The study helped clarifying which pdr mutants to use in studies of physiological actions of statins in yeast. | |||
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tumor protein p53 (TP53) | [2] | |||
| Resistant Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Expression | Rv3795; p.Glu378Ala |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| NCI- H460 cells | Pleural effusion | Homo sapiens (Human) | CVCL_0459 | |
| NCI-H1299 cells | Lymph node | Homo sapiens (Human) | CVCL_0060 | |
| NCI-H1355 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1464 | |
| CL1-0 cells | Lung | Homo sapiens (Human) | CVCL_3871 | |
| CL1-6 cells | Brain | Homo sapiens (Human) | N.A. | |
| Experiment for Drug Resistance |
MTT assay; Cell viability assay | |||
| Mechanism Description | Therefore, the drug sensitivity of DOC and lovastatin in human lung cancer cells was evaluated. We found that H1355 (mutant TP53-E285K), CL1 (mutant TP53-R248W), and H1299 (TP53-null) human non-small cell lung cancer cells were more sensitive to lovastatin than A549 and H460 cells expressing wild-type TP53. Conversely, A549 and H460 cells showed higher sensitivity to DOC than H1299 and CL1 cells, as demonstrated by the MTT assay. When endogenous TP53 activity was inhibited by pifithrin-alpha in A549 and H460 cells, lovastatin sensitivities significantly increased, and cancer cell viabilities markedly reduced. These results indicate that TP53 status is associated with the anti-cancer effect of statins in human lung cancer cells. Mutated or null TP53 status is correlated with higher statin sensitivity. Furthermore, DOC-resistant H1299 (H1299/D8) cells showed significant sensitivity to lovastatin treatment compared to DOC-resistant A549 (A549/D16) cells, indicating a potential application of statins/chemotherapy combination therapy to control wild-type and abnormal TP53-containing human lung tumors. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
