Molecule Information
General Information of the Molecule (ID: Mol00629)
| Name |
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
hSIRT1; NAD-dependent protein deacylase sirtuin-1; Regulatory protein SIR2 homolog 1; SIR2-like protein 1; hSIR2; 75SirT1; SIR2L1
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
SIRT1
|
||||
| Gene ID | |||||
| Location |
chr10:67884656-67918390[+]
|
||||
| Sequence |
MADEAALALQPGGSPSAAGADREAASSPAGEPLRKRPRRDGPGLERSPGEPGGAAPEREV
PAAARGCPGAAAAALWREAEAEAAAAGGEQEAQATAAAGEGDNGPGLQGPSREPPLADNL YDEDDDDEGEEEEEAAAAAIGYRDNLLFGDEIITNGFHSCESDEEDRASHASSSDWTPRP RIGPYTFVQQHLMIGTDPRTILKDLLPETIPPPELDDMTLWQIVINILSEPPKRKKRKDI NTIEDAVKLLQECKKIIVLTGAGVSVSCGIPDFRSRDGIYARLAVDFPDLPDPQAMFDIE YFRKDPRPFFKFAKEIYPGQFQPSLCHKFIALSDKEGKLLRNYTQNIDTLEQVAGIQRII QCHGSFATASCLICKYKVDCEAVRGDIFNQVVPRCPRCPADEPLAIMKPEIVFFGENLPE QFHRAMKYDKDEVDLLIVIGSSLKVRPVALIPSSIPHEVPQILINREPLPHLHFDVELLG DCDVIINELCHRLGGEYAKLCCNPVKLSEITEKPPRTQKELAYLSELPPTPLHVSEDSSS PERTSPPDSSVIVTLLDQAAKSNDDLDVSESKGCMEEKPQEVQTSRNVESIAEQMENPDL KNVGSSTGEKNERTSVAGTVRKCWPNRVAKEQISRRLDGNQYLFLPPNRYIFHGAEVYSD SEDDVLSSSSCGSNSDSGTCQSPSLEEPMEDESEIEEFYNGLEDEPDVPERAGGAGFGTD GDDQEAINEAISVKQEVTDMNYPSNKS Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
NAD-dependent protein deacetylase that links transcriptional regulation directly to intracellular energetics and participates in the coordination of several separated cellular functions such as cell cycle, response to DNA damage, metabolism, apoptosis and autophagy. Can modulate chromatin function through deacetylation of histones and can promote alterations in the methylation of histones and DNA, leading to transcriptional repression. Deacetylates a broad range of transcription factors and coregulators, thereby regulating target gene expression positively and negatively. Serves as a sensor of the cytosolic ratio of NAD(+)/NADH which is altered by glucose deprivation and metabolic changes associated with caloric restriction. Is essential in skeletal muscle cell differentiation and in response to low nutrients mediates the inhibitory effect on skeletal myoblast differentiation which also involves 5'-AMP-activated protein kinase (AMPK) and nicotinamide phosphoribosyltransferase (NAMPT). Component of the eNoSC (energy-dependent nucleolar silencing) complex, a complex that mediates silencing of rDNA in response to intracellular energy status and acts by recruiting histone-modifying enzymes. The eNoSC complex is able to sense the energy status of cell: upon glucose starvation, elevation of NAD(+)/NADP(+) ratio activates SIRT1, leading to histone H3 deacetylation followed by dimethylation of H3 at 'Lys-9' (H3K9me2) by SUV39H1 and the formation of silent chromatin in the rDNA locus. Deacetylates 'Lys-266' of SUV39H1, leading to its activation. Inhibits skeletal muscle differentiation by deacetylating PCAF and MYOD1. Deacetylates H2A and 'Lys-26' of H1-4. Deacetylates 'Lys-16' of histone H4 (in vitro). Involved in NR0B2/SHP corepression function through chromatin remodeling: Recruited to LRH1 target gene promoters by NR0B2/SHP thereby stimulating histone H3 and H4 deacetylation leading to transcriptional repression. Proposed to contribute to genomic integrity via positive regulation of telomere length; however, reports on localization to pericentromeric heterochromatin are conflicting. Proposed to play a role in constitutive heterochromatin (CH) formation and/or maintenance through regulation of the available pool of nuclear SUV39H1. Upon oxidative/metabolic stress decreases SUV39H1 degradation by inhibiting SUV39H1 polyubiquitination by MDM2. This increase in SUV39H1 levels enhances SUV39H1 turnover in CH, which in turn seems to accelerate renewal of the heterochromatin which correlates with greater genomic integrity during stress response. Deacetylates 'Lys-382' of p53/TP53 and impairs its ability to induce transcription-dependent proapoptotic program and modulate cell senescence. Deacetylates TAF1B and thereby represses rDNA transcription by the RNA polymerase I. Deacetylates MYC, promotes the association of MYC with MAX and decreases MYC stability leading to compromised transformational capability. Deacetylates FOXO3 in response to oxidative stress thereby increasing its ability to induce cell cycle arrest and resistance to oxidative stress but inhibiting FOXO3-mediated induction of apoptosis transcriptional activity; also leading to FOXO3 ubiquitination and protesomal degradation. Appears to have a similar effect on MLLT7/FOXO4 in regulation of transcriptional activity and apoptosis. Deacetylates DNMT1; thereby impairs DNMT1 methyltransferase-independent transcription repressor activity, modulates DNMT1 cell cycle regulatory function and DNMT1-mediated gene silencing. Deacetylates RELA/NF-kappa-B p65 thereby inhibiting its transactivating potential and augments apoptosis in response to TNF-alpha. Deacetylates HIF1A, KAT5/TIP60, RB1 and HIC1. Deacetylates FOXO1 resulting in its nuclear retention and enhancement of its transcriptional activity leading to increased gluconeogenesis in liver. Inhibits E2F1 transcriptional activity and apoptotic function, possibly by deacetylation. Involved in HES1- and HEY2-mediated transcriptional repression. In cooperation with MYCN seems to be involved in transcriptional repression of DUSP6/MAPK3 leading to MYCN stabilization by phosphorylation at 'Ser-62'. Deacetylates MEF2D. Required for antagonist-mediated transcription suppression of AR-dependent genes which may be linked to local deacetylation of histone H3. Represses HNF1A-mediated transcription. Required for the repression of ESRRG by CREBZF. Deacetylates NR1H3 and NR1H2 and deacetylation of NR1H3 at 'Lys-434' positively regulates transcription of NR1H3:RXR target genes, promotes NR1H3 proteosomal degradation and results in cholesterol efflux; a promoter clearing mechanism after reach round of transcription is proposed. Involved in lipid metabolism: deacetylates LPIN1, thereby inhibiting diacylglycerol synthesis. Implicated in regulation of adipogenesis and fat mobilization in white adipocytes by repression of PPARG which probably involves association with NCOR1 and SMRT/NCOR2. Deacetylates p300/EP300 and PRMT1. Deacetylates ACSS2 leading to its activation, and HMGCS1 deacetylation. Involved in liver and muscle metabolism. Through deacetylation and activation of PPARGC1A is required to activate fatty acid oxidation in skeletal muscle under low-glucose conditions and is involved in glucose homeostasis. Involved in regulation of PPARA and fatty acid beta-oxidation in liver. Involved in positive regulation of insulin secretion in pancreatic beta cells in response to glucose; the function seems to imply transcriptional repression of UCP2. Proposed to deacetylate IRS2 thereby facilitating its insulin-induced tyrosine phosphorylation. Deacetylates SREBF1 isoform SREBP-1C thereby decreasing its stability and transactivation in lipogenic gene expression. Involved in DNA damage response by repressing genes which are involved in DNA repair, such as XPC and TP73, deacetylating XRCC6/Ku70, and facilitating recruitment of additional factors to sites of damaged DNA, such as SIRT1-deacetylated NBN can recruit ATM to initiate DNA repair and SIRT1-deacetylated XPA interacts with RPA2. Also involved in DNA repair of DNA double-strand breaks by homologous recombination and specifically single-strand annealing independently of XRCC6/Ku70 and NBN. Transcriptional suppression of XPC probably involves an E2F4:RBL2 suppressor complex and protein kinase B (AKT) signaling. Transcriptional suppression of TP73 probably involves E2F4 and PCAF. Deacetylates WRN thereby regulating its helicase and exonuclease activities and regulates WRN nuclear translocation in response to DNA damage. Deacetylates APEX1 at 'Lys-6' and 'Lys-7' and stimulates cellular AP endonuclease activity by promoting the association of APEX1 to XRCC1. Catalyzes deacetylation of ERCC4/XPF, thereby impairing interaction with ERCC1 and nucleotide excision repair (NER). Increases p53/TP53-mediated transcription-independent apoptosis by blocking nuclear translocation of cytoplasmic p53/TP53 and probably redirecting it to mitochondria. Deacetylates XRCC6/Ku70 at 'Lys-539' and 'Lys-542' causing it to sequester BAX away from mitochondria thereby inhibiting stress-induced apoptosis. Is involved in autophagy, presumably by deacetylating ATG5, ATG7 and MAP1LC3B/ATG8. Deacetylates AKT1 which leads to enhanced binding of AKT1 and PDK1 to PIP3 and promotes their activation. Proposed to play role in regulation of STK11/LBK1-dependent AMPK signaling pathways implicated in cellular senescence which seems to involve the regulation of the acetylation status of STK11/LBK1. Can deacetylate STK11/LBK1 and thereby increase its activity, cytoplasmic localization and association with STRAD; however, the relevance of such activity in normal cells is unclear. In endothelial cells is shown to inhibit STK11/LBK1 activity and to promote its degradation. Deacetylates SMAD7 at 'Lys-64' and 'Lys-70' thereby promoting its degradation. Deacetylates CIITA and augments its MHC class II transactivation and contributes to its stability. Deacetylates MECOM/EVI1. Deacetylates PML at 'Lys-487' and this deacetylation promotes PML control of PER2 nuclear localization. During the neurogenic transition, represses selective NOTCH1-target genes through histone deacetylation in a BCL6-dependent manner and leading to neuronal differentiation. Regulates the circadian expression of several core clock genes, including ARNTL/BMAL1, RORC, PER2 and CRY1 and plays a critical role in maintaining a controlled rhythmicity in histone acetylation, thereby contributing to circadian chromatin remodeling. Deacetylates ARNTL/BMAL1 and histones at the circadian gene promoters in order to facilitate repression by inhibitory components of the circadian oscillator. Deacetylates PER2, facilitating its ubiquitination and degradation by the proteosome. Protects cardiomyocytes against palmitate-induced apoptosis. Deacetylates XBP1 isoform 2; deacetylation decreases protein stability of XBP1 isoform 2 and inhibits its transcriptional activity. Deacetylates PCK1 and directs its activity toward phosphoenolpyruvate production promoting gluconeogenesis. Involved in the CCAR2-mediated regulation of PCK1 and NR1D1. Deacetylates CTNB1 at 'Lys-49'. In POMC (pro-opiomelanocortin) neurons, required for leptin-induced activation of PI3K signaling. In addition to protein deacetylase activity, also acts as protein-lysine deacylase by mediating protein depropionylation and decrotonylation. Mediates depropionylation of Osterix (SP7). Catalyzes decrotonylation of histones; it however does not represent a major histone decrotonylase. Deacetylates SOX9; promoting SOX9 nuclear localization and transactivation activity. Involved in the regulation of centrosome duplication. Deacetylates CENATAC in G1 phase, allowing for SASS6 accumulation on the centrosome and subsequent procentriole assembly. Deacetylates NDC80/HEC1. {ECO:0000250|UniProtKB:Q923E4, ECO:0000269|11672523, ECO:0000269|12006491, ECO:0000269|12535671, ECO:0000269|14976264, ECO:0000269|14980222, ECO:0000269|15126506, ECO:0000269|15152190, ECO:0000269|15205477, ECO:0000269|15469825, ECO:0000269|15692560, ECO:0000269|16079181, ECO:0000269|16166628, ECO:0000269|16892051, ECO:0000269|16998810, ECO:0000269|17283066, ECO:0000269|17290224, ECO:0000269|17334224, ECO:0000269|17505061, ECO:0000269|17612497, ECO:0000269|17620057, ECO:0000269|17936707, ECO:0000269|18203716, ECO:0000269|18296641, ECO:0000269|18485871, ECO:0000269|18662546, ECO:0000269|18687677, ECO:0000269|19188449, ECO:0000269|19220062, ECO:0000269|19364925, ECO:0000269|19690166, ECO:0000269|19934257, ECO:0000269|20097625, ECO:0000269|20100829, ECO:0000269|20203304, ECO:0000269|20375098, ECO:0000269|20620956, ECO:0000269|20670893, ECO:0000269|20817729, ECO:0000269|20955178, ECO:0000269|21149730, ECO:0000269|21245319, ECO:0000269|21471201, ECO:0000269|21504832, ECO:0000269|21555002, ECO:0000269|21698133, ECO:0000269|21701047, ECO:0000269|21775285, ECO:0000269|21807113, ECO:0000269|21841822, ECO:0000269|21890893, ECO:0000269|21947282, ECO:0000269|22274616, ECO:0000269|24415752, ECO:0000269|24824780, ECO:0000269|28497810, ECO:0000269|29765047, ECO:0000269|30193097, ECO:0000269|30409912, ECO:0000269|31722219, ECO:0000269|32034146}.; FUNCTION: [Isoform 2]: Deacetylates 'Lys-382' of p53/TP53, however with lower activity than isoform 1. In combination, the two isoforms exert an additive effect. Isoform 2 regulates p53/TP53 expression and cellular stress response and is in turn repressed by p53/TP53 presenting a SIRT1 isoform-dependent auto-regulatory loop. {ECO:0000269|20975832}.; FUNCTION: (Microbial infection) In case of HIV-1 infection, interacts with and deacetylates the viral Tat protein. The viral Tat protein inhibits SIRT1 deacetylation activity toward RELA/NF-kappa-B p65, thereby potentiates its transcriptional activity and SIRT1 is proposed to contribute to T-cell hyperactivation during infection. {ECO:0000269|18329615}.; FUNCTION: [SirtT1 75 kDa fragment]: Catalytically inactive 75SirT1 may be involved in regulation of apoptosis. May be involved in protecting chondrocytes from apoptotic death by associating with cytochrome C and interfering with apoptosome assembly. {ECO:0000269|21987377}.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
4 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [1] | |||
| Sensitive Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Gastric cancer [ICD-11: 2B72] | |||
| The Specified Disease | Gastric cancer | |||
| The Studied Tissue | Gastric tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.90E-02 Fold-change: 6.03E-02 Z-score: 4.00E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | SIRT1/CREB/ABCG2 signaling pathway | Regulation | N.A. | |
| In Vitro Model | MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 |
| MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Upregulated miR132 in Lgr5+ gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Overexpression of SIRT1 down-regulated ABCG2 expression by promoting the de-acetylation of the transcription factor CREB. CREB was further activated ABCG2 via binding to the promoter of ABCG2 to induce transcription. | |||
|
|
||||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [4] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.37E-06 Fold-change: -2.94E-02 Z-score: -4.63E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SBC5 cells | Lung | Homo sapiens (Human) | CVCL_1679 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Cells pretreated with siR-Sirt1 are more sensitive to DDP than the control pretreated cells, miR-34a down-regulation Sirt1 and sensitizes lung cancer cell lines to DDP. | |||
| Disease Class: Bladder cancer [ICD-11: 2C94.0] | [5] | |||
| Sensitive Disease | Bladder cancer [ICD-11: 2C94.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Bladder cancer [ICD-11: 2C94] | |||
| The Specified Disease | Bladder cancer | |||
| The Studied Tissue | Bladder tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.20E-11 Fold-change: -1.85E-01 Z-score: -1.20E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell proliferation | Inhibition | hsa05200 | |
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| TCCSuP cells | Bladder | Homo sapiens (Human) | CVCL_1738 | |
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
WST-1 assay | |||
| Mechanism Description | Cdk6, in complex with Cdk4 and cyclin D1, is a key regulator of Rb activity and thereby G1/S transition, SIRT-1 is a deacetylase whose targets including p53, FOXO, SFRP1 and PGC1. Transfection with pre-miR-34a increases chemo-sensitivity to cisplatin through inhibition of Cdk6 and SIRT-1. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colon cancer [ICD-11: 2B90.1] | [2] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Colon cancer [ICD-11: 2B90] | |||
| The Specified Disease | Colon cancer | |||
| The Studied Tissue | Colon tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.34E-02 Fold-change: 1.46E-02 Z-score: 2.28E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | PI3K/AKT signaling pathway | Activation | hsa04151 | |
| In Vitro Model | DLD1 cells | Colon | Homo sapiens (Human) | CVCL_0248 |
| DLD-1/5FU cells | Colon | Homo sapiens (Human) | CVCL_0248 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | The ectopic expression of miR-34a in the 5-FU-resistant cells inhibited growth, as in the parental cells, and attenuated the resistance to 5-FU through the down-regulation of Sirt1 and E2F3. | |||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [7] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell proliferation | Activation | hsa05200 | ||
| miR4766-5p/SIRT1 signaling pathway | Regulation | N.A. | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| MDA-MB-468 cells | Breast | Homo sapiens (Human) | CVCL_0419 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; PE Annexin V assay; TUNEL assay; Invasion assay | |||
| Mechanism Description | The promotion of cell proliferation and metastasis, the acquisition of chemoresistance, and the increased expression of SIRT1 induced by LncRNA-PRLB overexpression could be partly abrogated by ectopic expression of miR-4766-5p. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [6] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | UCA1/miR204/Sirt1 signaling pathway | Activation | hsa05206 | |
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 | |
| PNT2 cells | Prostate | Homo sapiens (Human) | CVCL_2164 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Annexin V-FITC Apoptosis assay; Flow cytometer | |||
| Mechanism Description | The UCA1/miR204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. miR204 negatively modulated Sirt1 expression in prostate cancer cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [8] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell viability | Inhibition | hsa05200 | |
| In Vitro Model | PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | SIRT1 plays crucial roles in various cellular processes including cell survival under genotoxic and oxidative stresses. Bcl2I is an anti-apoptotic factor. In PC3PR cells, reduced expression of miR-34a confers paclitaxel resistance via up-regulating SIRT1 and Bcl2 expression. | |||
Clinical Trial Drug(s)
1 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [3] | |||
| Sensitive Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Sensitive Drug | Camptothecin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.32E-01 Fold-change: -1.01E-02 Z-score: -3.46E-01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell growth | Inhibition | hsa05200 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | DU-145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 |
| LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 | |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| PrEC cells | Prostate | Homo sapiens (Human) | CVCL_0061 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Trypan blue dye exclusion assay | |||
| Mechanism Description | Inhibition of the SIRT1 activity or expression resulted in attenuation of cell proliferation and chemoresistance in PC3 and DU145 cells. Ectopic expression of miR-34a decreased the SIRT1 mRNA and protein levels as well as protein levels of known direct target genes. Ectopic miR-34a expression resulted in cell cycle arrest and growth inhibition and attenuated chemoresistance to anticancer drug camptothecin by inducing apoptosis. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.90E-02; Fold-change: 3.83E-01; Z-score: 2.68E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.49E-02; Fold-change: 1.68E-01; Z-score: 3.85E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
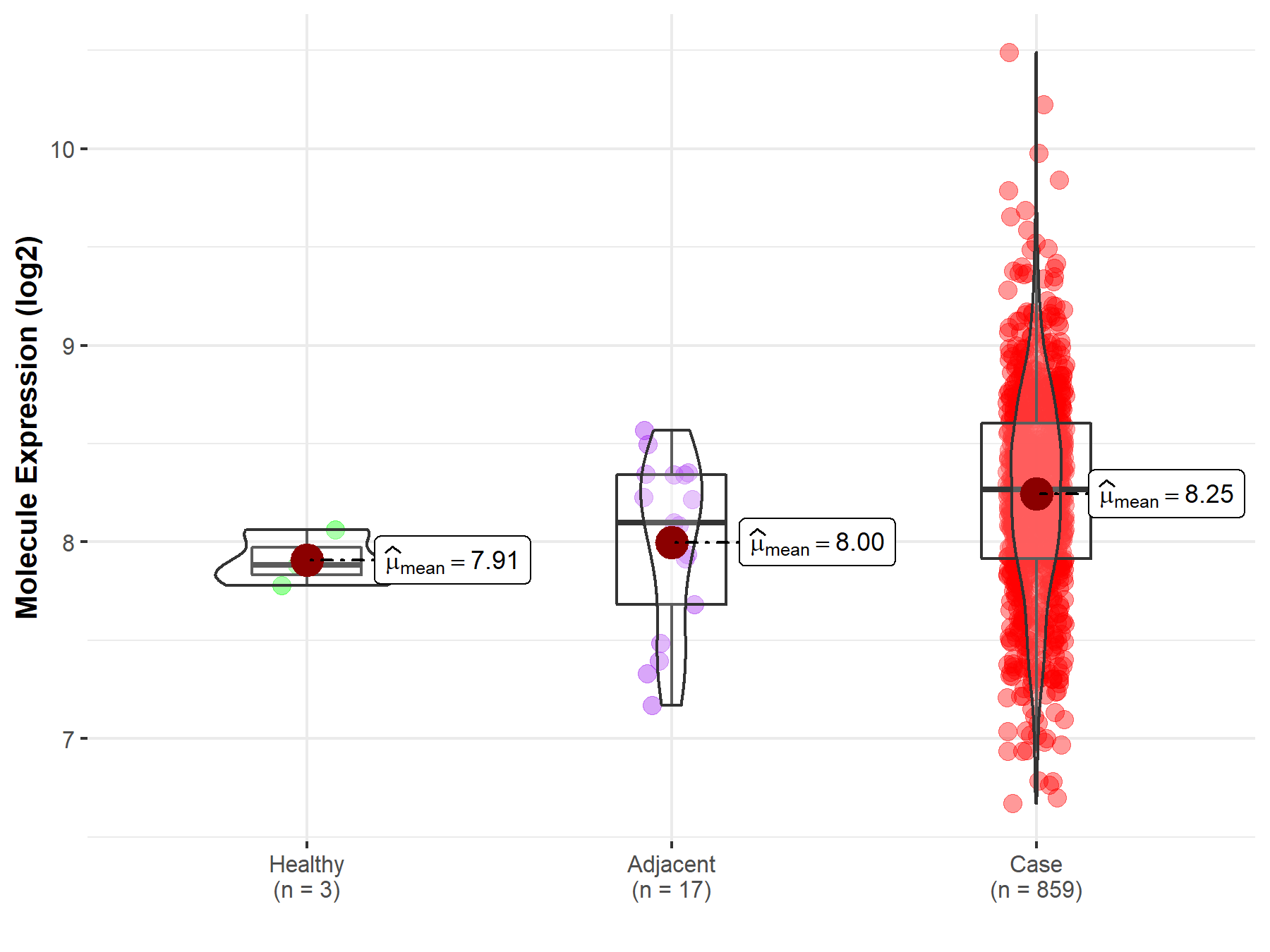
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Colon | |
| The Specified Disease | Colon cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.34E-02; Fold-change: 7.89E-02; Z-score: 2.10E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.13E-13; Fold-change: -3.56E-01; Z-score: -5.96E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
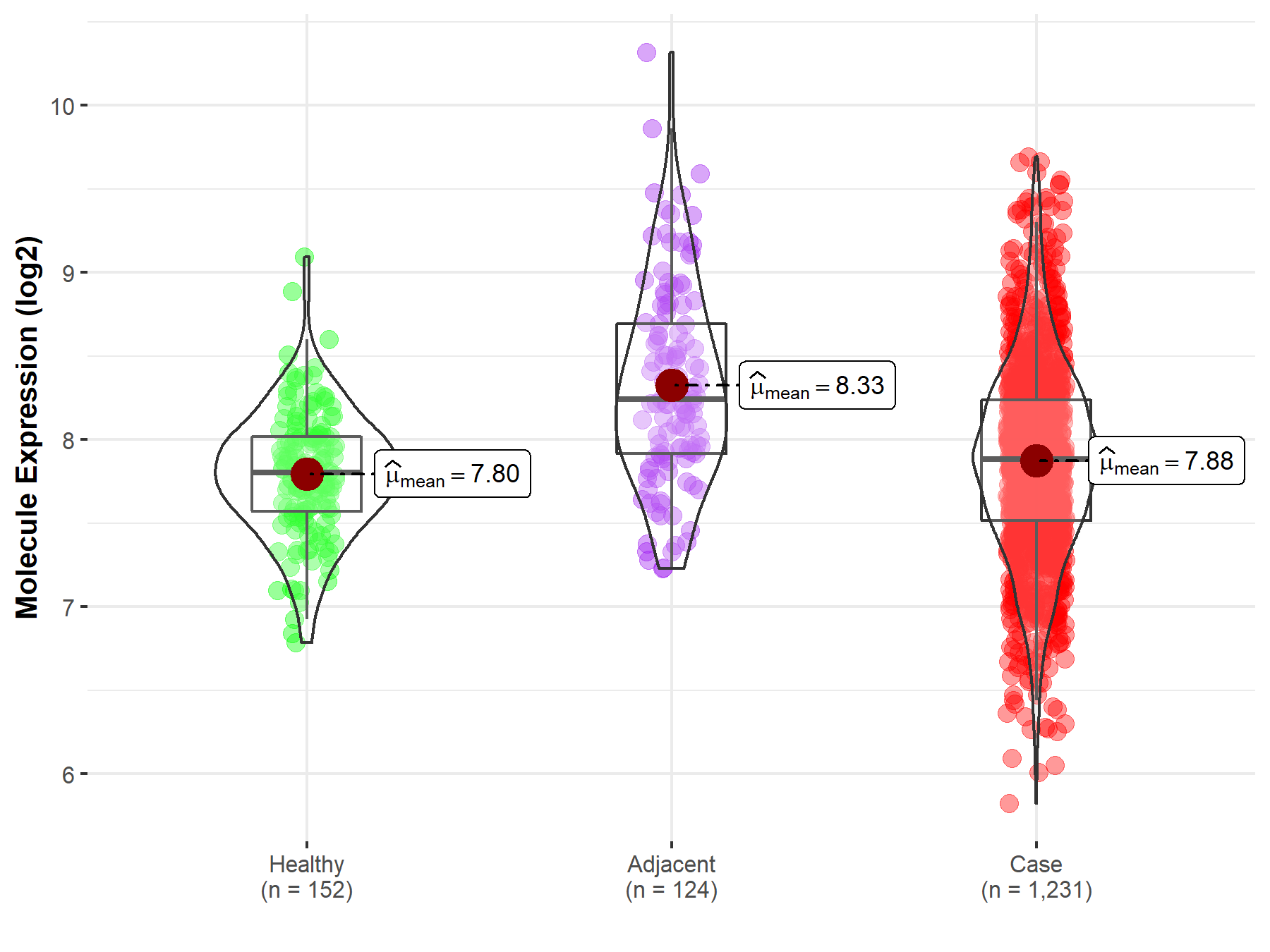
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.37E-06; Fold-change: -1.87E-01; Z-score: -4.47E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.50E-10; Fold-change: -2.43E-01; Z-score: -5.09E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
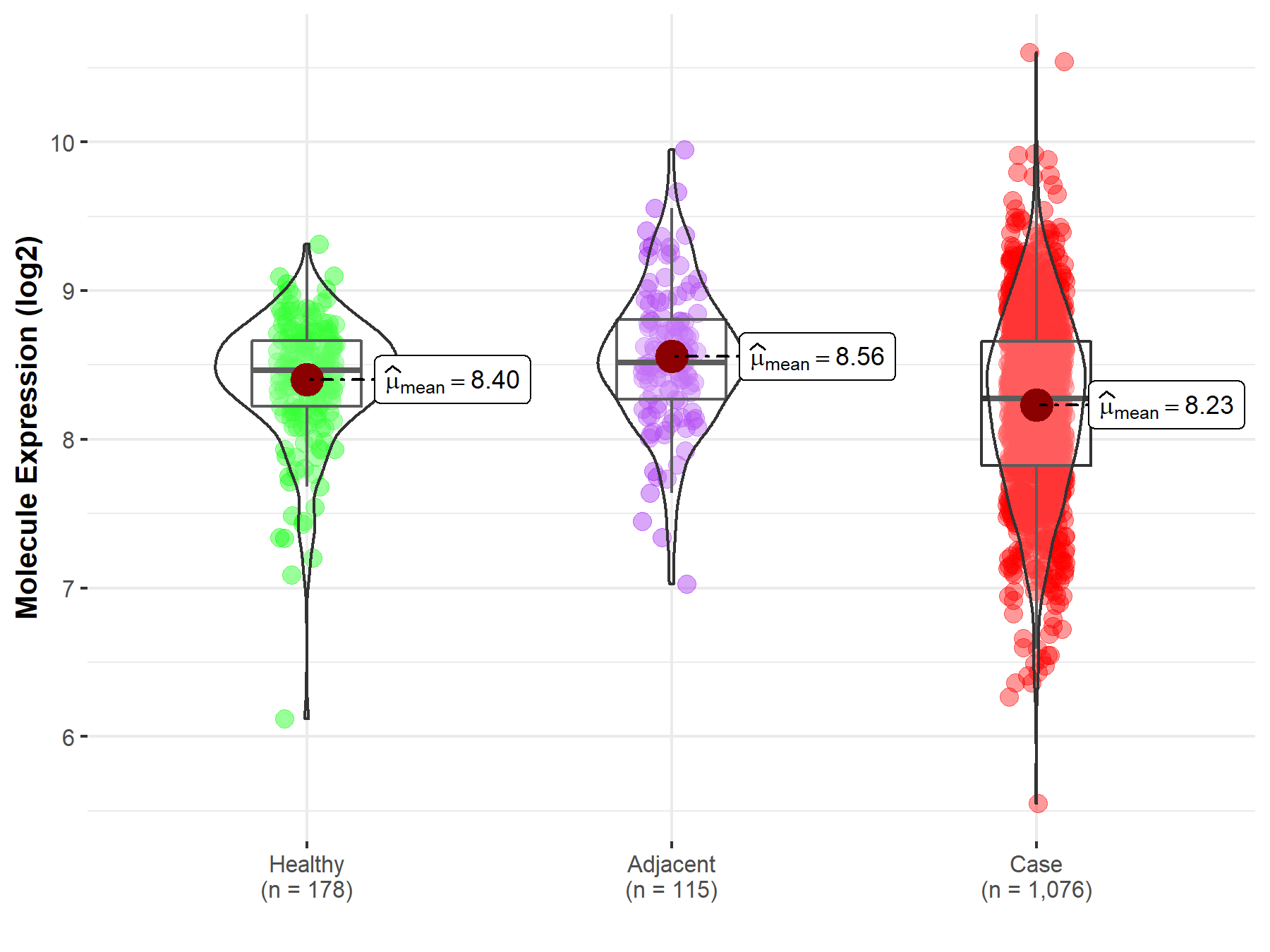
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.19E-09; Fold-change: -1.92E-01; Z-score: -3.41E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.43E-02; Fold-change: -3.19E-01; Z-score: -5.49E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
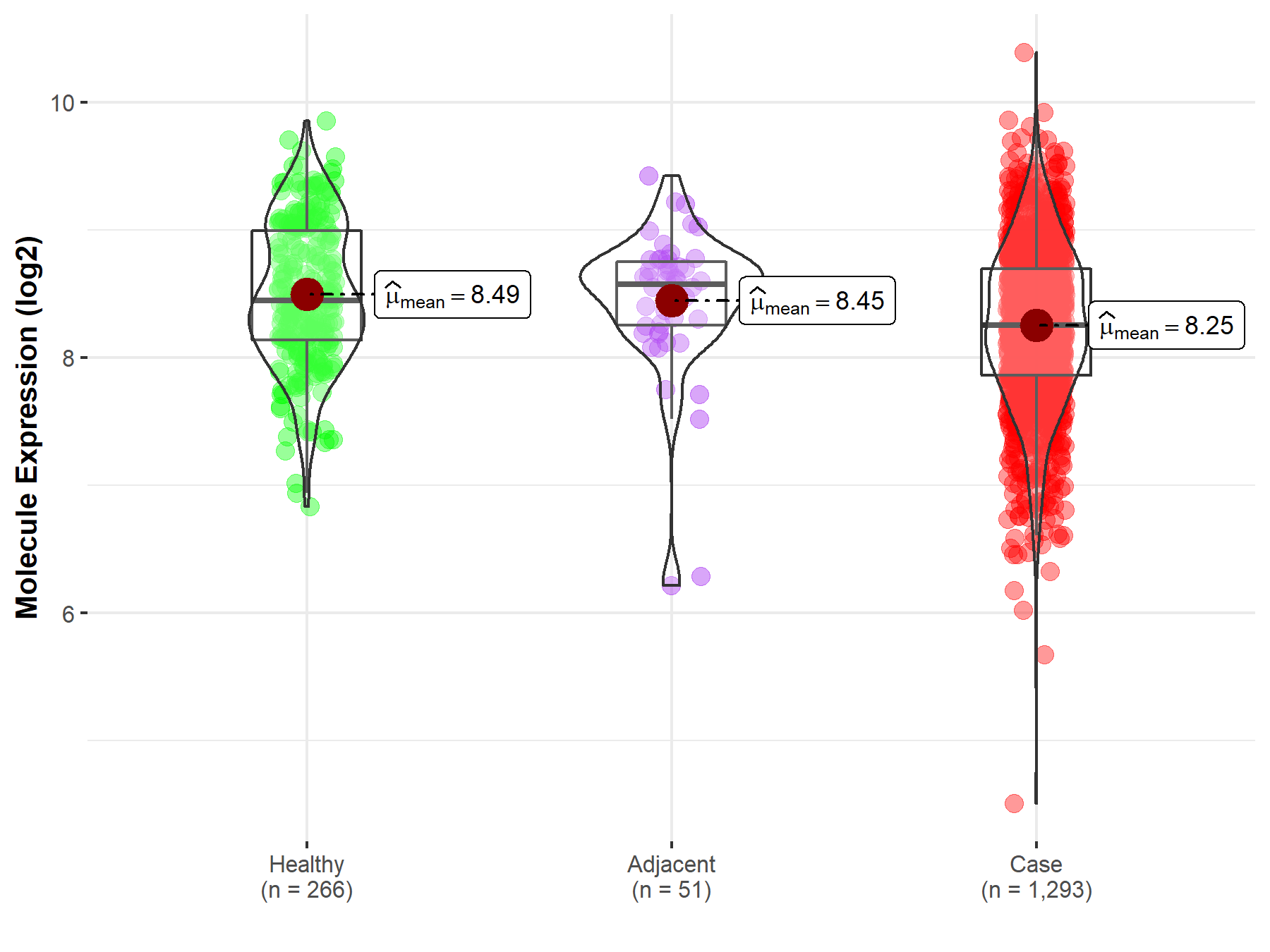
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.32E-01; Fold-change: 1.29E-01; Z-score: 1.97E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
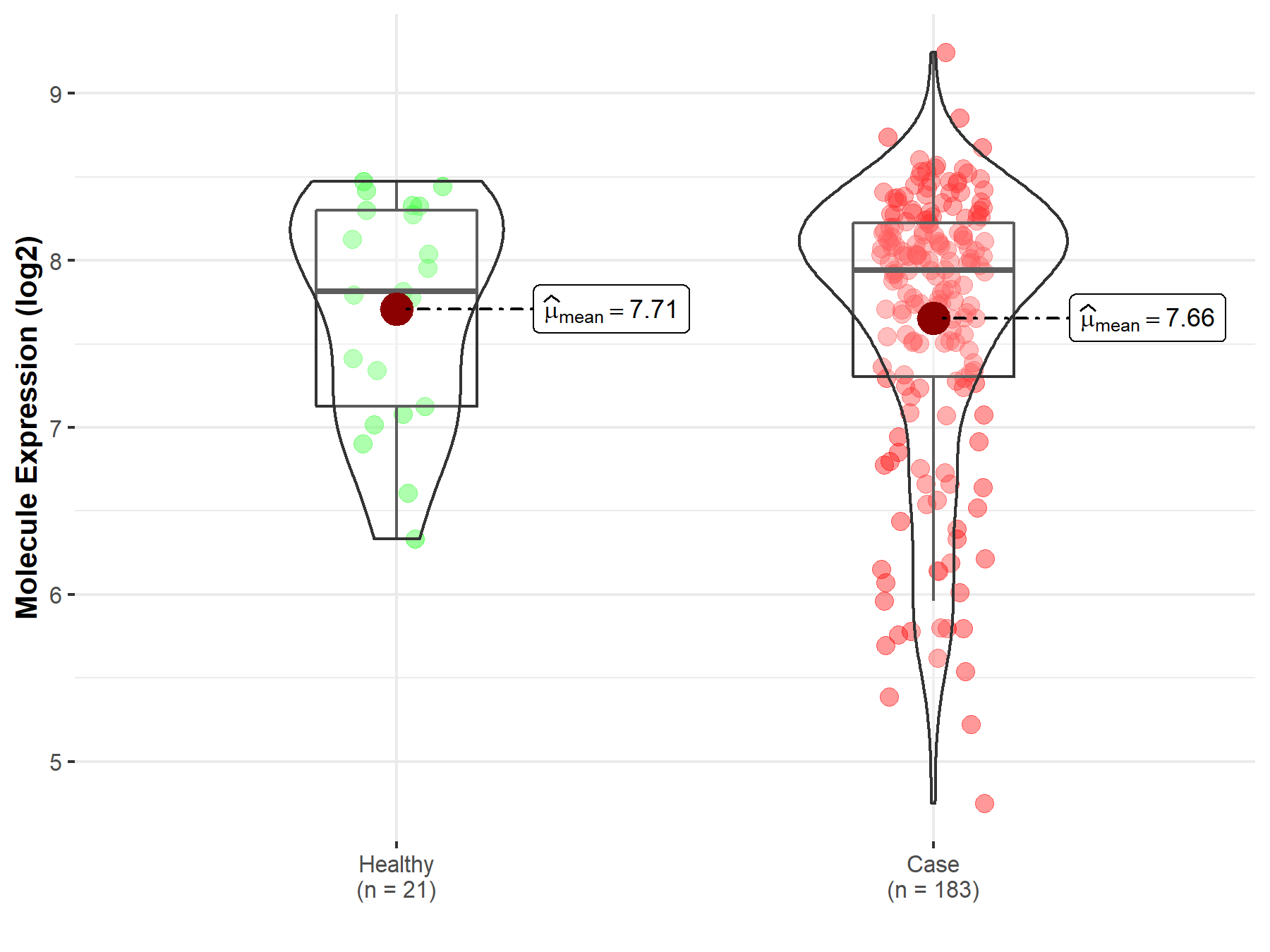
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.20E-11; Fold-change: -1.22E+00; Z-score: -6.68E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
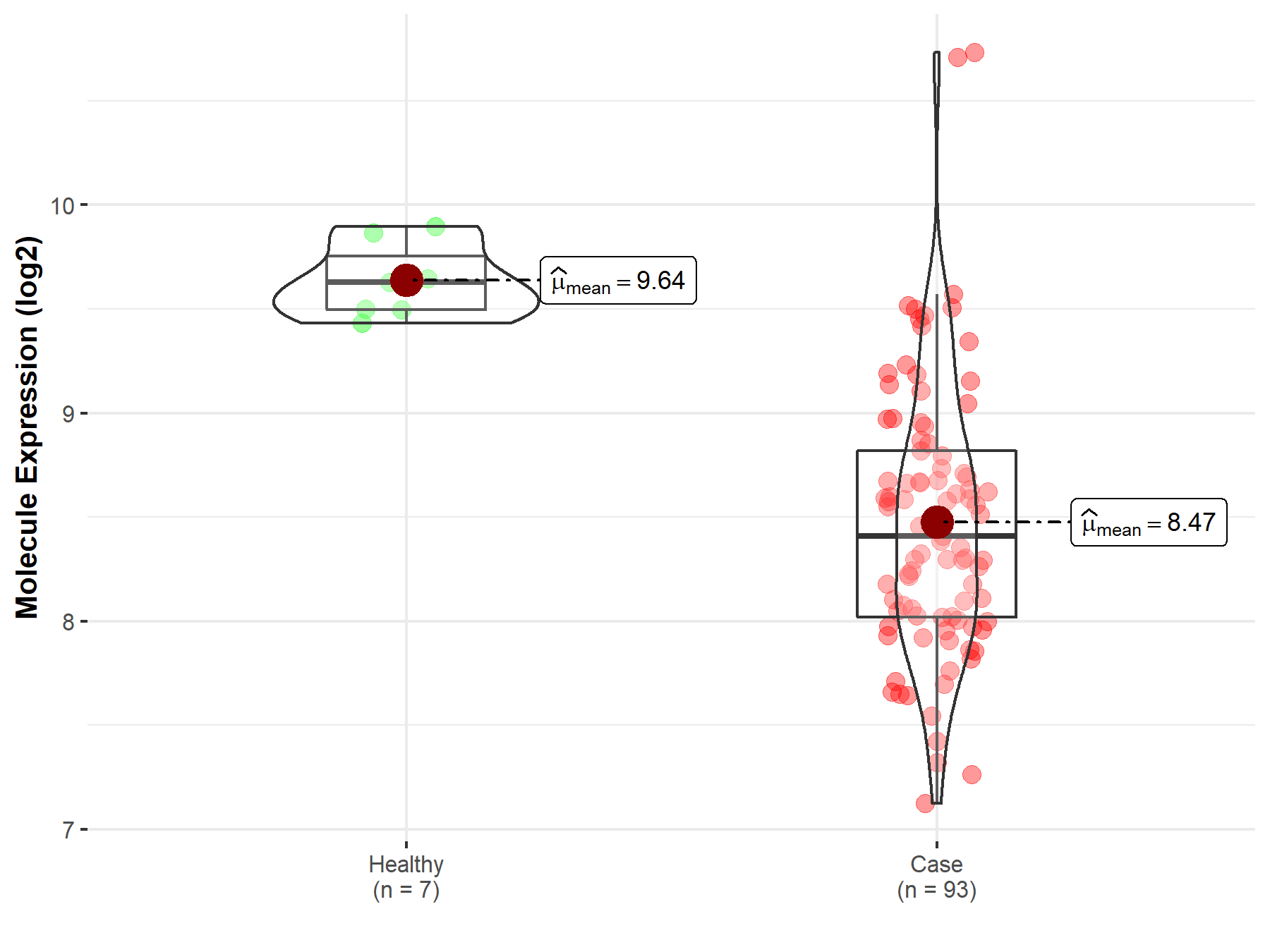
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

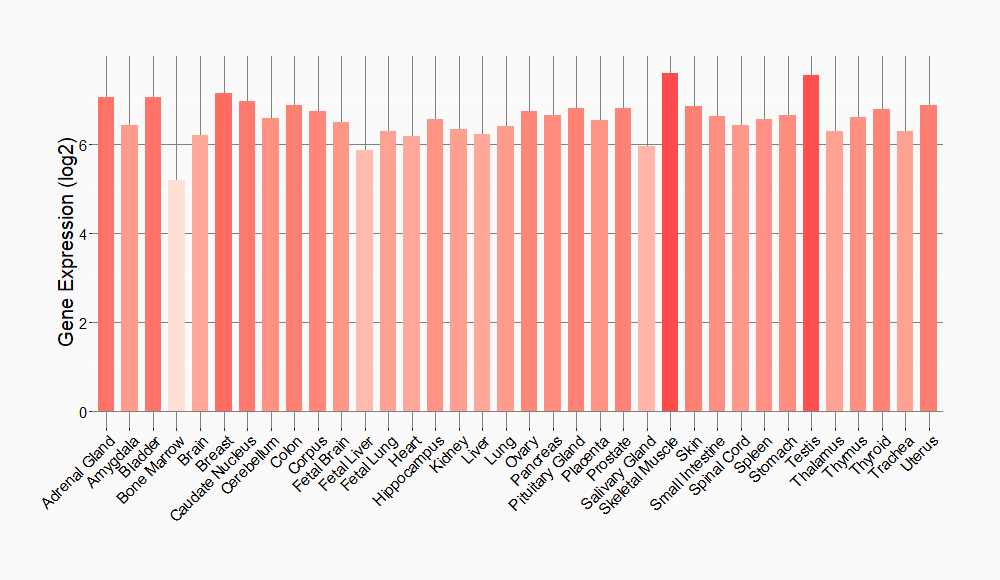
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
