Molecule Information
General Information of the Molecule (ID: Mol00267)
| Name |
Caspase-3 (CASP3)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
CASP-3; Apopain; Cysteine protease CPP32; CPP-32; Protein Yama; SREBP cleavage activity 1; SCA-1; CPP32
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
CASP3
|
||||
| Gene ID | |||||
| Location |
chr4:184627696-184649509[-]
|
||||
| Sequence |
MENTENSVDSKSIKNLEPKIIHGSESMDSGISLDNSYKMDYPEMGLCIIINNKNFHKSTG
MTSRSGTDVDAANLRETFRNLKYEVRNKNDLTREEIVELMRDVSKEDHSKRSSFVCVLLS HGEEGIIFGTNGPVDLKKITNFFRGDRCRSLTGKPKLFIIQACRGTELDCGIETDSGVDD DMACHKIPVEADFLYAYSTAPGYYSWRNSKDGSWFIQSLCAMLKQYADKLEFMHILTRVN RKVATEFESFSFDATFHAKKQIPCIVSMLTKELYFYH Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Involved in the activation cascade of caspases responsible for apoptosis execution. At the onset of apoptosis it proteolytically cleaves poly(ADP-ribose) polymerase (PARP) at a '216-Asp-|-Gly-217' bond. Cleaves and activates sterol regulatory element binding proteins (SREBPs) between the basic helix-loop-helix leucine zipper domain and the membrane attachment domain. Cleaves and activates caspase-6, -7 and -9. Involved in the cleavage of huntingtin. Triggers cell adhesion in sympathetic neurons through RET cleavage. Cleaves and inhibits serine/threonine-protein kinase AKT1 in response to oxidative stress. Acts as an inhibitor of type I interferon production during virus-induced apoptosis by mediating cleavage of antiviral proteins CGAS, IRF3 and MAVS, thereby preventing cytokine overproduction. Cleaves XRCC4 and phospholipid scramblase proteins XKR4, XKR8 and XKR9, leading to promote phosphatidylserine exposure on apoptotic cell surface.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
6 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Lung cancer [ICD-11: 2C25.5] | [1] | |||
| Sensitive Disease | Lung cancer [ICD-11: 2C25.5] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Lung cancer [ICD-11: 2C25] | |||
| The Specified Disease | Lung cancer | |||
| The Studied Tissue | Lung tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.09E-54 Fold-change: 1.02E-01 Z-score: 1.86E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | AKT/ERK signaling pathway | Inhibition | hsa04010 | |
| Cell apoptosis | Activation | hsa04210 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Suppression of miR-155 in this cell line considerably reversed doxorubicin resistance, and doxorubicin-induced apoptosis and cell cycle arrest were recovered. Furthermore, reverse transcription-polymerase chain reaction and western blot analysis revealed that miR-155 suppression downregulated the expression of multidrug resistance protein 1, multidrug resistance-associated protein 1, breast cancer resistance protein, glutathione S-transferase-Pi, Survivin and B-cell lymphoma 2, and upregulated the expression of caspase-3 and caspase-8. In addition, it was found that miR-155 suppression inhibited the activation of AkT and extracellular signal-regulated kinase. The transcriptional activity of nuclear factor-kB and activator protein-1 was also downregulated. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [6] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [6] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | [6] | |||
| Resistant Disease | Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Cervical cancer [ICD-11: 2C77.0] | [2] | |||
| Resistant Disease | Cervical cancer [ICD-11: 2C77.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | Hela cells | Cervix uteri | Homo sapiens (Human) | CVCL_0030 |
| HeLa/DDP cells | Uterus | Homo sapiens (Human) | CVCL_C869 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; EdU assay; Flow cytometric analysis | |||
| Mechanism Description | UCA1 suppressed apoptosis by downregulating caspase 3 and upregulating CDk2, whereas enhanced cell proliferation by increased level of survivin and decreased level of p21. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [3] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell invasion | Activation | hsa05200 | ||
| Cell migration | Activation | hsa04670 | ||
| N-Myc/ miR421 /ATM signaling pathway | Regulation | N.A. | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| HEK293T cells | Kidney | Homo sapiens (Human) | CVCL_0063 | |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| GES-1 cells | Gastric | Homo sapiens (Human) | CVCL_EQ22 | |
| HGC27 cells | Gastric | Homo sapiens (Human) | CVCL_1279 | |
| NCI-N87 cells | Gastric | Homo sapiens (Human) | CVCL_1603 | |
| MkN-45 cells | Gastric | Homo sapiens (Human) | CVCL_0434 | |
| MkN28 cells | Gastric | Homo sapiens (Human) | CVCL_1416 | |
| SNU-16 cells | Gastric | Homo sapiens (Human) | CVCL_0076 | |
| Experiment for Molecule Alteration |
Western blot analysis; Flow cytometric assay | |||
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | Overexpression of miR-421 promoted metastasis, inhibited apoptosis, and induced cisplatin resistance in gastric cancer by targeting E-cadherin and caspase-3. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [4] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | A2780/DDP cells | Ovary | Homo sapiens (Human) | CVCL_D619 |
| SkOV-3/DDP cells | Ovary | Homo sapiens (Human) | CVCL_UI88 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometry assay | |||
| Mechanism Description | PVT1 was overexpressed in tumor tissues of cisplatin-resistant patients comparing to cisplatin-sensitive patients. PVT1 knockdown significantly lowered cell viability and increased the percentage of apoptotic tumor cells in SkOV-3/DDP and A2780/DDP cells transfected with siPVT1 and treated with cisplatin. It manifested PVT1 knockdown can reverses the cisplatin resistance in cisplatin-resistant cell lines. | |||
| Disease Class: Gastric cancer [ICD-11: 2B72.1] | [5] | |||
| Resistant Disease | Gastric cancer [ICD-11: 2B72.1] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell proliferation | Activation | hsa05200 | ||
| Notch1 signaling pathway | Activation | hsa04330 | ||
| In Vitro Model | SGC7901 cells | Gastric | Homo sapiens (Human) | CVCL_0520 |
| BGC823 cells | Gastric | Homo sapiens (Human) | CVCL_3360 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating LncRNA Ak022798 expression. First, we found that Notch 1 was highly expressed in the cisplatin-resistant gastric cancer cell lines SGC7901/DDP and BGC823/DDP cells. Furthermore, we used siRNA to interfere with LncRNA Ak022798 expression, and found that the expression of MRP1 and P-glycoprotein decreased significantly in SGC7901/DDP and BGC823/DDP cells, and their apoptosis as well as the expressions of caspase 3 and caspase 8 obviously increased. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [6] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Resistant Drug | Lamivudine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [6] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Lamivudine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | [6] | |||
| Resistant Disease | Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | |||
| Resistant Drug | Lamivudine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast adenocarcinoma [ICD-11: 2C60.1] | [6] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [6] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Disease Class: Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | [6] | |||
| Resistant Disease | Cutaneous squamous cell carcinoma [ICD-11: 2C31.1] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | A431 cells | Skin | Homo sapiens (Human) | CVCL_0037 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Caspase-3 is the key executioner caspase in apoptosis. Ectopic expression of let-7adecreased the luciferase activity of a reporter constructcontaining the 30untranslated region of caspase-3. Enforced let-7aexpression increased the resistance in A431 cells andHepG2 cells to apoptosis induced by therapeutic drugs suchas interferon-gamma, doxorubicin and paclitaxel. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hepatocellular carcinoma [ICD-11: 2C12.2] | [7] | |||
| Resistant Disease | Hepatocellular carcinoma [ICD-11: 2C12.2] | |||
| Resistant Drug | Sorafenib | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| In Vitro Model | Huh-7 cells | Liver | Homo sapiens (Human) | CVCL_0336 |
| HepG2 cells | Liver | Homo sapiens (Human) | CVCL_0027 | |
| Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 | |
| PLC/PRF/5 cells | Liver | Homo sapiens (Human) | CVCL_0485 | |
| SNU182 cells | Liver | Homo sapiens (Human) | CVCL_0090 | |
| SNU398 cells | Liver | Homo sapiens (Human) | CVCL_0077 | |
| SNU449 cells | Liver | Homo sapiens (Human) | CVCL_0454 | |
| SNU475 cells | Liver | Homo sapiens (Human) | CVCL_0497 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Caspase 3/7 activity assay; Cell-titer-Glo assay; Flow cytometry assay | |||
| Mechanism Description | In hepatocellular carcinoma miR221 modulates sorafenib resistance through inhibition of caspase-3-mediated apoptosis. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Hereditary angioedema [ICD-11: 4B05.0] | [8] | |||
| Resistant Disease | Hereditary angioedema [ICD-11: 4B05.0] | |||
| Resistant Drug | Stanozolol | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Sprague Dawley male rats model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
Gene expression analysis | |||
| Mechanism Description | Stanozolol can increases levels of Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio. Resistance training, 50 and 100 mg/kg Tribulus terrestris and resistance training along with Tribulus terrestris can decrease the Bax, Bcl-2, P53, caspase 3 and Bax/Bcl-2 ratio in rats exposed to Stanozolol. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Gastric tissue | |
| The Specified Disease | Gastric cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.47E-01; Fold-change: 6.64E-01; Z-score: 6.70E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.31E-02; Fold-change: -1.79E-01; Z-score: -3.36E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
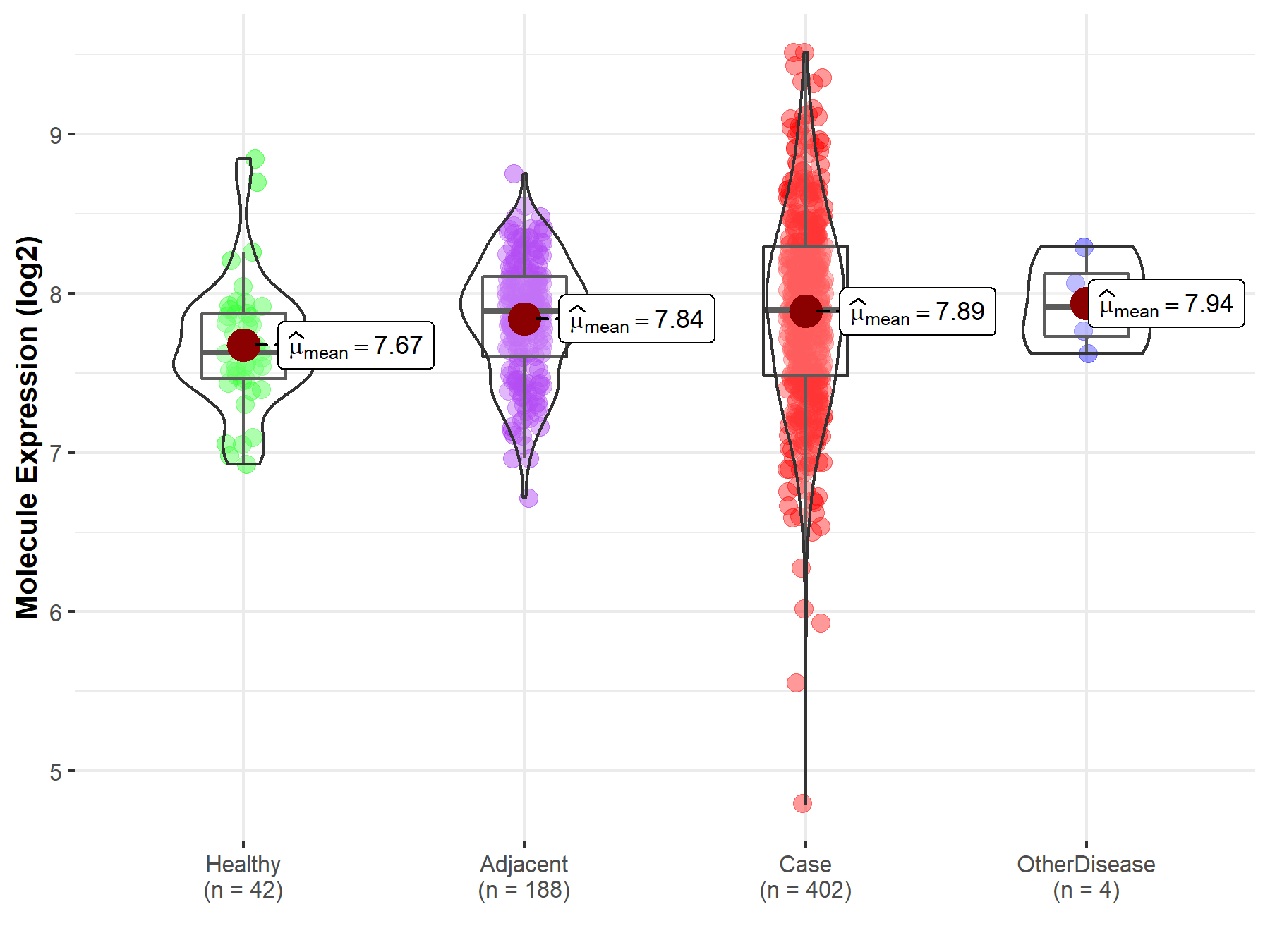
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Liver | |
| The Specified Disease | Liver cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.03E-03; Fold-change: 2.66E-01; Z-score: 6.68E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.70E-01; Fold-change: 8.05E-03; Z-score: 2.16E-02 | |
| The Expression Level of Disease Section Compare with the Other Disease Section | p-value: 7.64E-01; Fold-change: -2.17E-02; Z-score: -7.18E-02 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
Molecule expression in tissue other than the diseased tissue of patients
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
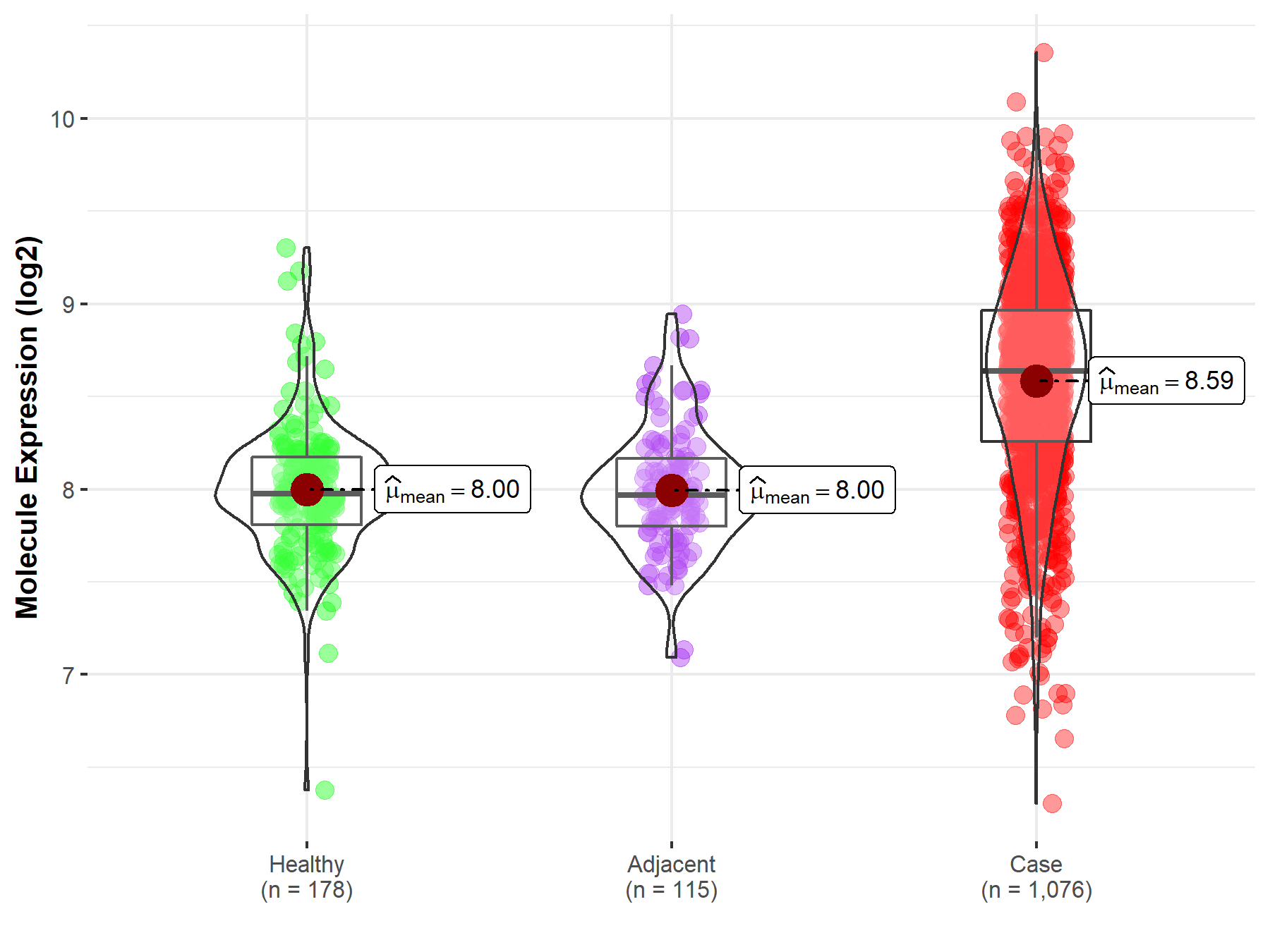
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.09E-54; Fold-change: 6.61E-01; Z-score: 1.88E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 5.15E-40; Fold-change: 6.71E-01; Z-score: 2.06E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Skin | |
| The Specified Disease | Skin squamous cell carcinoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.89E-29; Fold-change: 4.35E-01; Z-score: 1.20E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 1.43E-35; Fold-change: 4.69E-01; Z-score: 1.21E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
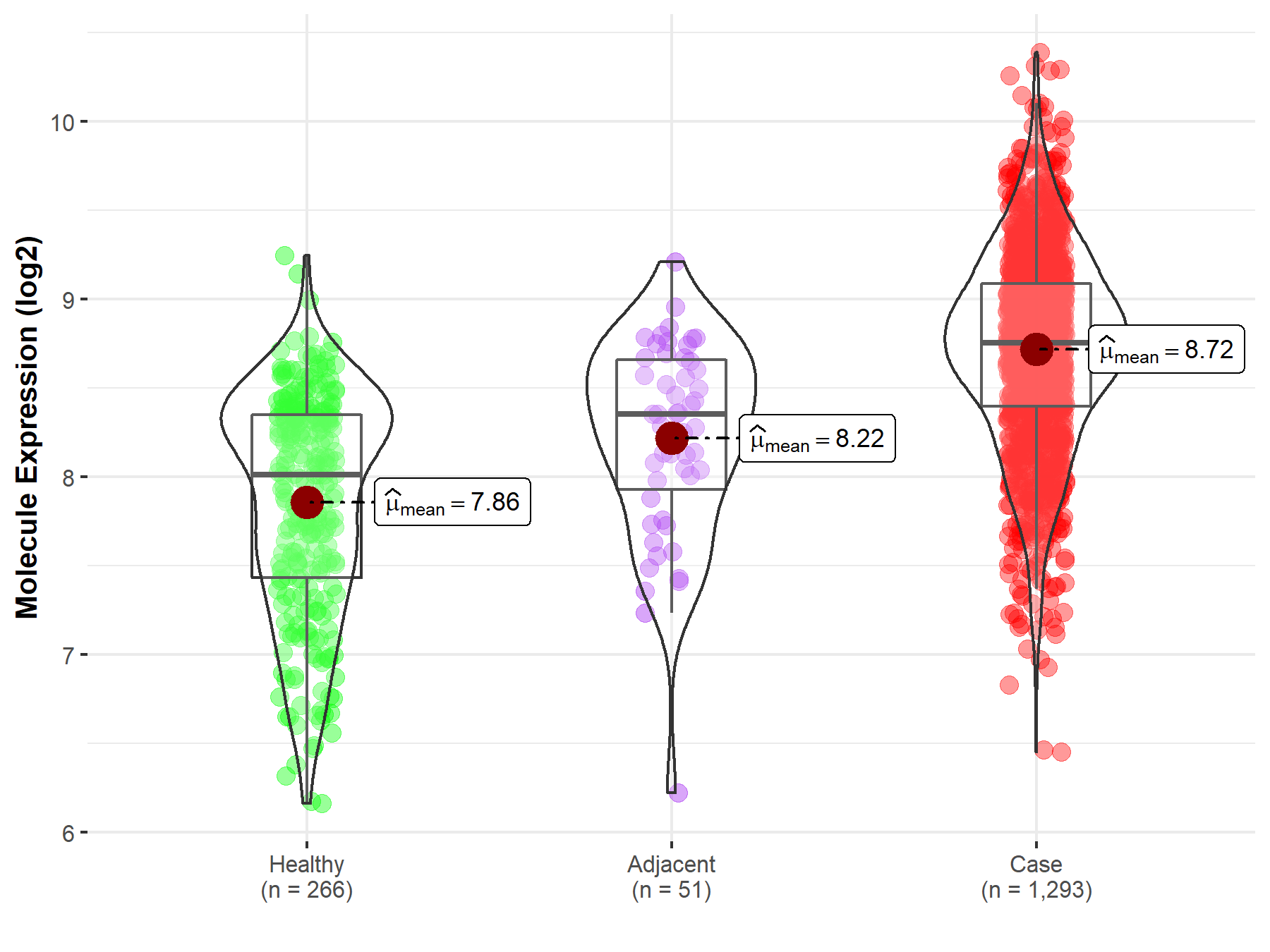
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.06E-64; Fold-change: 7.42E-01; Z-score: 1.19E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.89E-08; Fold-change: 4.00E-01; Z-score: 7.22E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.56E-05; Fold-change: 7.86E-01; Z-score: 2.40E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 6.81E-02; Fold-change: -3.88E-01; Z-score: -4.48E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
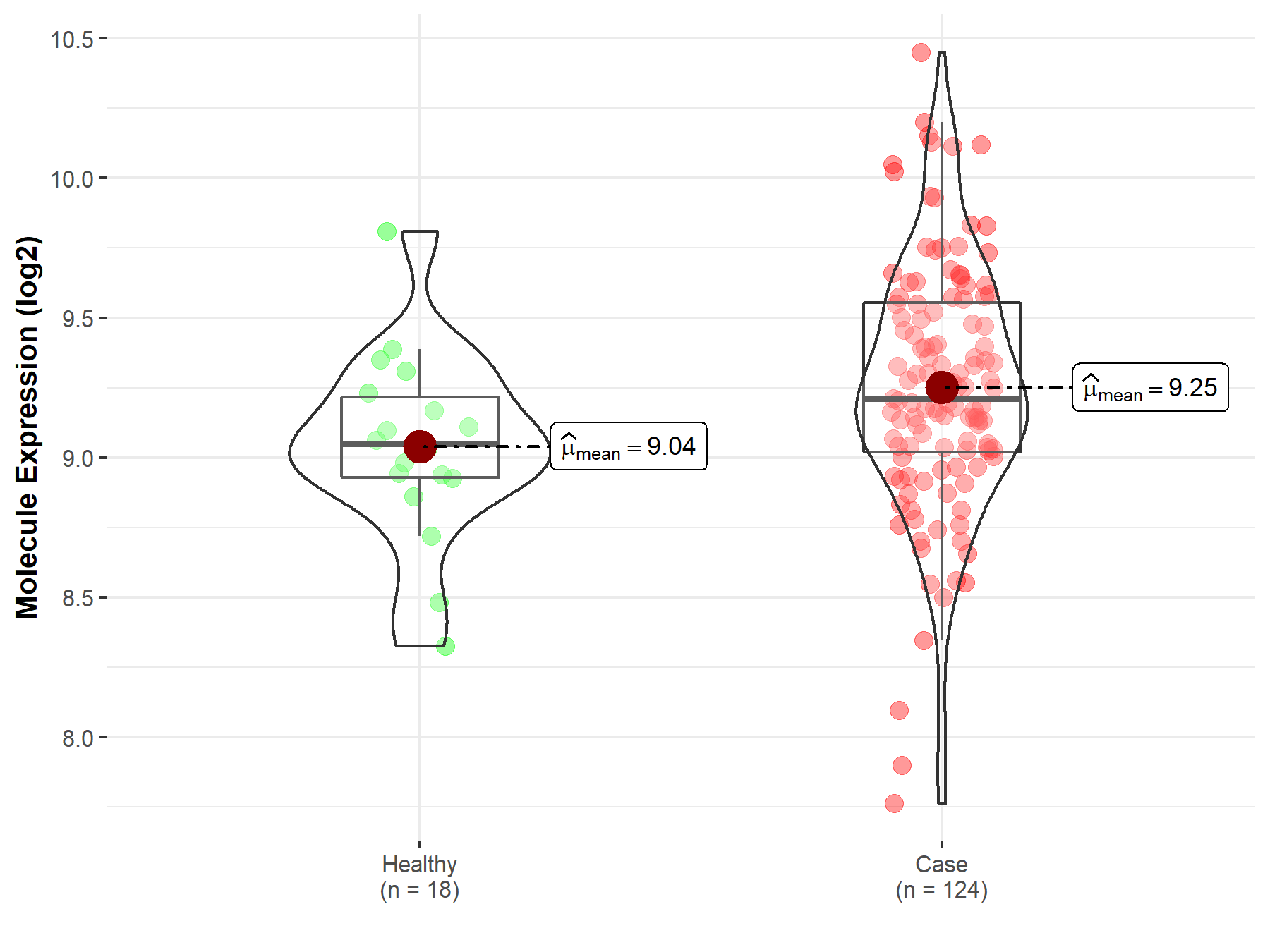
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Cervix uteri | |
| The Specified Disease | Cervical cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.60E-02; Fold-change: 1.61E-01; Z-score: 4.77E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals


|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
