Molecule Information
General Information of the Molecule (ID: Mol00169)
| Name |
Transcription factor SOX-2 (SOX2)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Molecule Type |
Protein
|
||||
| Gene Name |
SOX2
|
||||
| Gene ID | |||||
| Location |
chr3:181711925-181714436[+]
|
||||
| Sequence |
MYNMMETELKPPGPQQTSGGGGGNSTAAAAGGNQKNSPDRVKRPMNAFMVWSRGQRRKMA
QENPKMHNSEISKRLGAEWKLLSETEKRPFIDEAKRLRALHMKEHPDYKYRPRRKTKTLM KKDKYTLPGGLLAPGGNSMASGVGVGAGLGAGVNQRMDSYAHMNGWSNGSYSMMQDQLGY PQHPGLNAHGAAQMQPMHRYDVSALQYNSMTSSQTYMNGSPTYSMSYSQQGTPGMALGSM GSVVKSEASSSPPVVTSSSHSRAPCQAGDLRDMISMYLPGAEVPEPAAPSRLHMSQHYQS GPVPGTAINGTLPLSHM Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Transcription factor that forms a trimeric complex with OCT4 on DNA and controls the expression of a number of genes involved in embryonic development such as YES1, FGF4, UTF1 and ZFP206. Binds to the proximal enhancer region of NANOG. Critical for early embryogenesis and for embryonic stem cell pluripotency. Downstream SRRT target that mediates the promotion of neural stem cell self-renewal. Keeps neural cells undifferentiated by counteracting the activity of proneural proteins and suppresses neuronal differentiation. May function as a switch in neuronal development.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
7 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Sarcoma [ICD-11: 2C35.0] | [1] | |||
| Resistant Disease | Sarcoma [ICD-11: 2C35.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Sarcoma [ICD-11: 2C35] | |||
| The Specified Disease | Sarcoma | |||
| The Studied Tissue | Muscle tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.02E-14 Fold-change: 8.51E-02 Z-score: 7.66E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SW-872 cells | Skin | Homo sapiens (Human) | CVCL_1730 |
| SW-1353 cells | Brain | Homo sapiens (Human) | CVCL_0543 | |
| TE-671 cells | Peripheral blood | Homo sapiens (Human) | CVCL_1756 | |
| SW-684 cells | Skin | Homo sapiens (Human) | CVCL_1726 | |
| SW-982 cells | Testicular | Homo sapiens (Human) | CVCL_1734 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | By investigating of important regulators of stem cell biology, real-time RT-PCR data showed an increased expression of c-Myc, beta-catenin, and SOX-2 in the ALDH1high population and a significant higher level of ABCG2. Statistical analysis of data demonstrated that ALDH1high cells of SW-982 and SW-1353 showed higher resistance to commonly used chemotherapeutic agents like doxorubicin, epirubicin, and cisplatin than ALDH1low cells. This study demonstrates that in different sarcoma cell lines, high ALDH1 activity can be used to identify a subpopulation of cells characterized by a significantly higher proliferation rate, increased colony forming, increased expression of ABC transporter genes and stemness markers compared to control cells. In addition, enhanced drug resistance was demonstrated. | |||
|
|
||||
| Disease Class: Bladder urothelial carcinoma [ICD-11: 2C94.2] | [2] | |||
| Resistant Disease | Bladder urothelial carcinoma [ICD-11: 2C94.2] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| BFTC 909 cells | Kidney | Homo sapiens (Human) | CVCL_1084 | |
| BFTC 905 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1083 | |
| HT-1376 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1292 | |
| SCaBER cells | Urinary bladder | Homo sapiens (Human) | CVCL_3599 | |
| RT-4 cells | Urinary bladder | Homo sapiens (Human) | CVCL_0036 | |
| UM-UC3 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1783 | |
| In Vivo Model | Athymic (nu+/nu+) mouse xenograft model; NOD/SCID/IL2Rgamma -/- mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Mechanism Description | Chemotherapy-induced COX2 and YAP1 signaling may promote CSC expansion via SOX2 overexpression and subsequent chemotherapy resistance.The YAP1-SOX2 axis, via re-activated PI3K/AKT signaling, may also be relevant to an acquired resistance to the EGFR inhibitor, as demonstrated by our findings that the resistant tumors again became sensitive to the EGFR inhibitor in combination with the YAP1 inhibitor. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Bladder urothelial carcinoma [ICD-11: 2C94.2] | [2] | |||
| Resistant Disease | Bladder urothelial carcinoma [ICD-11: 2C94.2] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Bladder cancer [ICD-11: 2C94] | |||
| The Specified Disease | Bladder cancer | |||
| The Studied Tissue | Bladder tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.57E-02 Fold-change: 6.55E-02 Z-score: 2.25E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | 5637 cells | Bladder | Homo sapiens (Human) | CVCL_0126 |
| J82 cells | Bladder | Homo sapiens (Human) | CVCL_0359 | |
| T24 cells | Bladder | Homo sapiens (Human) | CVCL_0554 | |
| BFTC 909 cells | Kidney | Homo sapiens (Human) | CVCL_1084 | |
| BFTC 905 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1083 | |
| HT-1376 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1292 | |
| SCaBER cells | Urinary bladder | Homo sapiens (Human) | CVCL_3599 | |
| RT-4 cells | Urinary bladder | Homo sapiens (Human) | CVCL_0036 | |
| UM-UC3 cells | Urinary bladder | Homo sapiens (Human) | CVCL_1783 | |
| In Vivo Model | Athymic (nu+/nu+) mouse xenograft model; NOD/SCID/IL2Rgamma -/- mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blotting assay | |||
| Mechanism Description | Chemotherapy-induced COX2 and YAP1 signaling may promote CSC expansion via SOX2 overexpression and subsequent chemotherapy resistance.The YAP1-SOX2 axis, via re-activated PI3K/AKT signaling, may also be relevant to an acquired resistance to the EGFR inhibitor, as demonstrated by our findings that the resistant tumors again became sensitive to the EGFR inhibitor in combination with the YAP1 inhibitor. | |||
|
|
||||
| Disease Class: Pancreatic cancer [ICD-11: 2C10.3] | [7] | |||
| Resistant Disease | Pancreatic cancer [ICD-11: 2C10.3] | |||
| Resistant Drug | Gemcitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| Cell proliferation | Activation | hsa05200 | ||
| In Vitro Model | AsPC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0152 |
| CFPAC1 cells | Pancreas | Homo sapiens (Human) | CVCL_1119 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | MALAT-1 could increase the proportion of pancreatic CSCs, maintain self-renewing capacity, decrease the chemosensitivity to anticancer drugs, and accelerate tumor angiogenesis in vitro, and promote tumorigenicity of pancreatic cancer cells in vivo. The underlying mechanisms may involve in increased expression of self-renewal related factors Sox2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [3] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Resistant Drug | Paclitaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.42E-05 Fold-change: 2.55E-02 Z-score: 4.37E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | Down-regulation of miR 200c 3p contributes to the resistance of breast cancer cells to paclitaxel by targeting SOX2. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Glioblastoma [ICD-11: 2A00.02] | [4] | |||
| Sensitive Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Sensitive Drug | Temozolomide | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Neuroectodermal tumor | |||
| The Studied Tissue | Brainstem tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.12E-04 Fold-change: -1.08E-01 Z-score: -3.80E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell apoptosis | Inhibition | hsa04210 | ||
| Wnt/Beta-catenin signaling pathway | Activation | hsa04310 | ||
| In Vitro Model | U251 cells | Brain | Homo sapiens (Human) | CVCL_0021 |
| U87 cells | Brain | Homo sapiens (Human) | CVCL_0022 | |
| Experiment for Molecule Alteration |
Western blot analysis; RT-qPCR | |||
| Experiment for Drug Resistance |
CCK8 assay; Colony formation assay; Flow cytometry assay | |||
| Mechanism Description | miR-126-3p sensitizes glioblastoma cells to temozolomide by inactivating Wnt/beta-catenin signaling via targeting SOX2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Sarcoma [ICD-11: 2C35.0] | [1] | |||
| Resistant Disease | Sarcoma [ICD-11: 2C35.0] | |||
| Resistant Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SW-872 cells | Skin | Homo sapiens (Human) | CVCL_1730 |
| SW-1353 cells | Brain | Homo sapiens (Human) | CVCL_0543 | |
| TE-671 cells | Peripheral blood | Homo sapiens (Human) | CVCL_1756 | |
| SW-684 cells | Skin | Homo sapiens (Human) | CVCL_1726 | |
| SW-982 cells | Testicular | Homo sapiens (Human) | CVCL_1734 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | By investigating of important regulators of stem cell biology, real-time RT-PCR data showed an increased expression of c-Myc, beta-catenin, and SOX-2 in the ALDH1high population and a significant higher level of ABCG2. Statistical analysis of data demonstrated that ALDH1high cells of SW-982 and SW-1353 showed higher resistance to commonly used chemotherapeutic agents like doxorubicin, epirubicin, and cisplatin than ALDH1low cells. This study demonstrates that in different sarcoma cell lines, high ALDH1 activity can be used to identify a subpopulation of cells characterized by a significantly higher proliferation rate, increased colony forming, increased expression of ABC transporter genes and stemness markers compared to control cells. In addition, enhanced drug resistance was demonstrated. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Breast cancer [ICD-11: 2C60.3] | [5] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Sensitive Drug | Doxorubicin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell colony | Inhibition | hsa05200 | ||
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-231 cells | Breast | Homo sapiens (Human) | CVCL_0062 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; RIP assay; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
CCK8; Colony formation assay; wound healing; Transwell invasion; Flow cytometry assay | |||
| Mechanism Description | miR-129-5p suppresses Adriamycin resistance in breast cancer by directly targeting SOX2. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Sarcoma [ICD-11: 2C35.0] | [1] | |||
| Resistant Disease | Sarcoma [ICD-11: 2C35.0] | |||
| Resistant Drug | Epirubicin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SW-872 cells | Skin | Homo sapiens (Human) | CVCL_1730 |
| SW-1353 cells | Brain | Homo sapiens (Human) | CVCL_0543 | |
| TE-671 cells | Peripheral blood | Homo sapiens (Human) | CVCL_1756 | |
| SW-684 cells | Skin | Homo sapiens (Human) | CVCL_1726 | |
| SW-982 cells | Testicular | Homo sapiens (Human) | CVCL_1734 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | By investigating of important regulators of stem cell biology, real-time RT-PCR data showed an increased expression of c-Myc, beta-catenin, and SOX-2 in the ALDH1high population and a significant higher level of ABCG2. Statistical analysis of data demonstrated that ALDH1high cells of SW-982 and SW-1353 showed higher resistance to commonly used chemotherapeutic agents like doxorubicin, epirubicin, and cisplatin than ALDH1low cells. This study demonstrates that in different sarcoma cell lines, high ALDH1 activity can be used to identify a subpopulation of cells characterized by a significantly higher proliferation rate, increased colony forming, increased expression of ABC transporter genes and stemness markers compared to control cells. In addition, enhanced drug resistance was demonstrated. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Colorectal cancer [ICD-11: 2B91.1] | [6] | |||
| Resistant Disease | Colorectal cancer [ICD-11: 2B91.1] | |||
| Resistant Drug | Fluorouracil | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell proliferation | Activation | hsa05200 | |
| In Vitro Model | SW480 cells | Colon | Homo sapiens (Human) | CVCL_0546 |
| SW620 cells | Colon | Homo sapiens (Human) | CVCL_0547 | |
| HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |
| HT-29 cells | Colon | Homo sapiens (Human) | CVCL_0320 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-450b-5p inhibited stemness and development of chemoresistance to 5-FU by targeting SOX2 in CRC cells. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Nervous tissue | |
| The Specified Disease | Brain cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.56E-14; Fold-change: 4.49E-01; Z-score: 6.09E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.24E-01; Fold-change: 1.16E+00; Z-score: 1.23E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | White matter | |
| The Specified Disease | Glioma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.52E-07; Fold-change: 9.05E-01; Z-score: 2.47E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Brainstem tissue | |
| The Specified Disease | Neuroectodermal tumor | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.12E-04; Fold-change: -1.32E-01; Z-score: -3.78E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
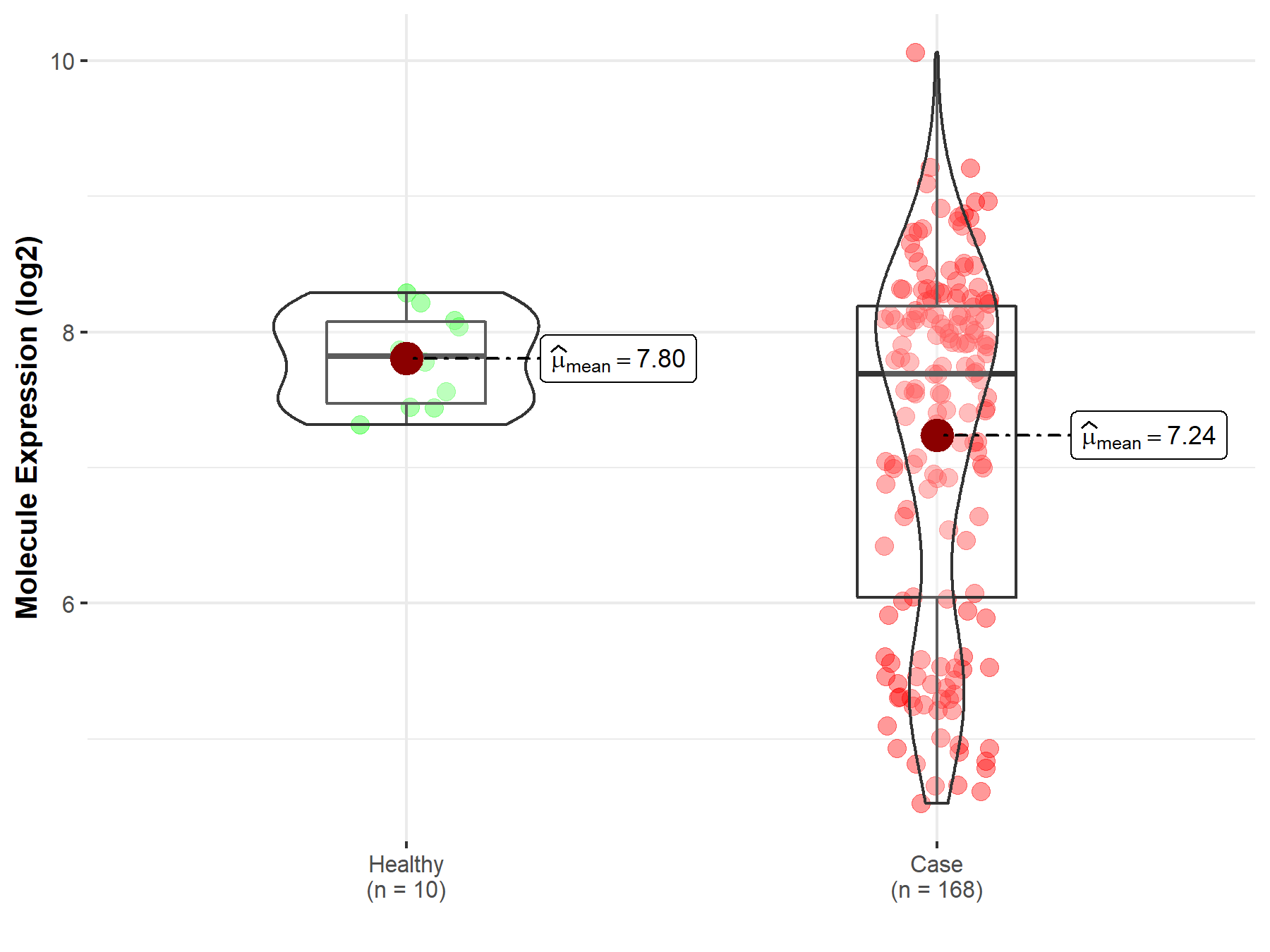
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Pancreas | |
| The Specified Disease | Pancreatic cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.61E-02; Fold-change: -2.38E-01; Z-score: -7.26E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 8.32E-03; Fold-change: -2.59E-01; Z-score: -7.01E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Muscle | |
| The Specified Disease | Sarcoma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 7.02E-14; Fold-change: 1.04E-02; Z-score: 5.22E-02 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 5.35E-02; Fold-change: -1.10E-01; Z-score: -1.18E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Breast tissue | |
| The Specified Disease | Breast cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.42E-05; Fold-change: 1.01E-02; Z-score: 4.44E-02 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 3.18E-02; Fold-change: -5.68E-02; Z-score: -2.36E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bladder tissue | |
| The Specified Disease | Bladder cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.57E-02; Fold-change: 2.29E-01; Z-score: 6.80E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
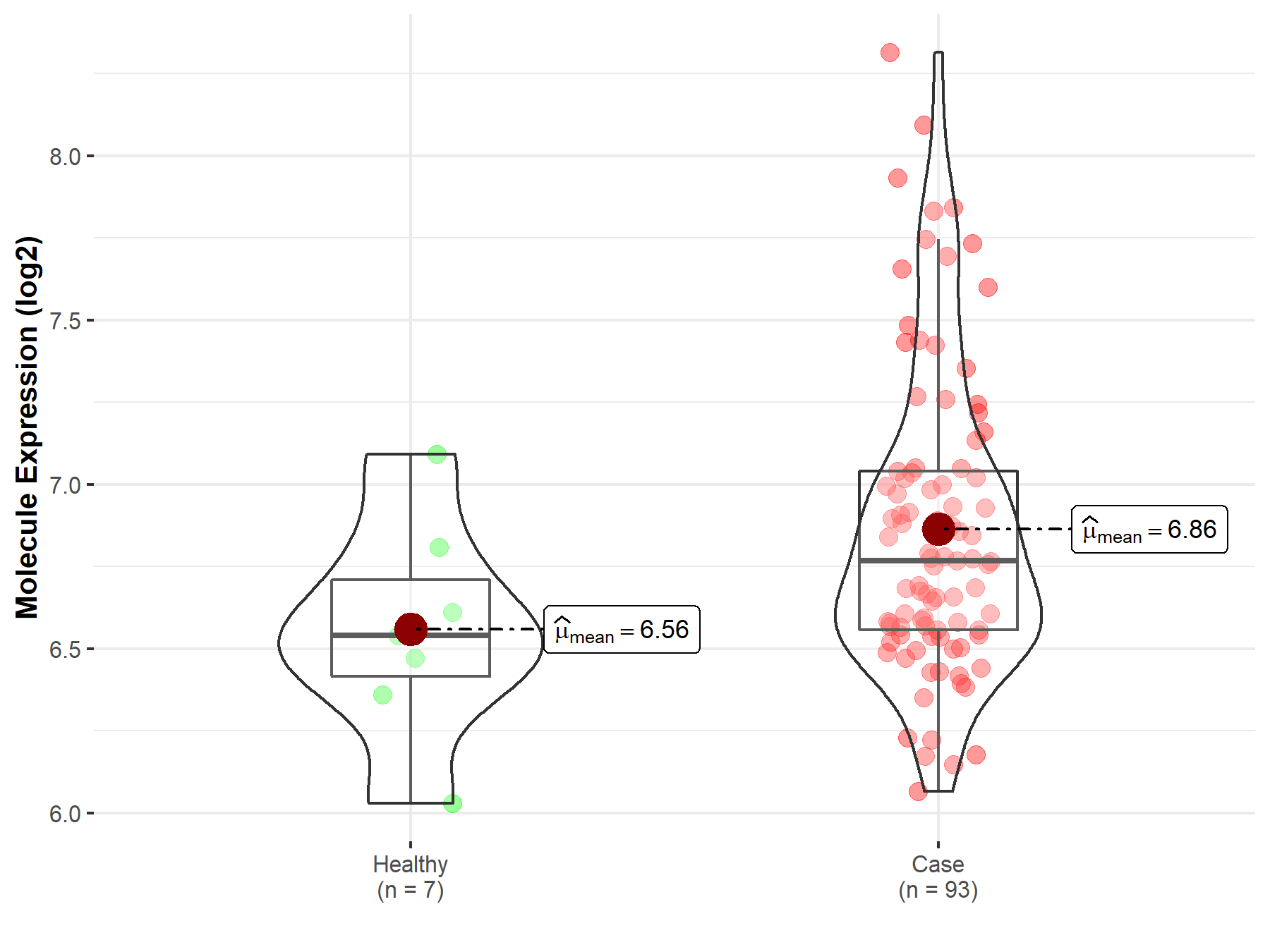
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals


|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
