Molecule Information
General Information of the Molecule (ID: Mol00058)
| Name |
DNA (cytosine-5)-methyltransferase 1 (DNMT1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
Dnmt1; CXXC-type zinc finger protein 9; DNA methyltransferase HsaI; DNA MTase HsaI; M.HsaI; MCMT; AIM; CXXC9; DNMT
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
DNMT1
|
||||
| Gene ID | |||||
| Location |
chr19:10133342-10231286[-]
|
||||
| Sequence |
MPARTAPARVPTLAVPAISLPDDVRRRLKDLERDSLTEKECVKEKLNLLHEFLQTEIKNQ
LCDLETKLRKEELSEEGYLAKVKSLLNKDLSLENGAHAYNREVNGRLENGNQARSEARRV GMADANSPPKPLSKPRTPRRSKSDGEAKPEPSPSPRITRKSTRQTTITSHFAKGPAKRKP QEESERAKSDESIKEEDKDQDEKRRRVTSRERVARPLPAEEPERAKSGTRTEKEEERDEK EEKRLRSQTKEPTPKQKLKEEPDREARAGVQADEDEDGDEKDEKKHRSQPKDLAAKRRPE EKEPEKVNPQISDEKDEDEKEEKRRKTTPKEPTEKKMARAKTVMNSKTHPPKCIQCGQYL DDPDLKYGQHPPDAVDEPQMLTNEKLSIFDANESGFESYEALPQHKLTCFSVYCKHGHLC PIDTGLIEKNIELFFSGSAKPIYDDDPSLEGGVNGKNLGPINEWWITGFDGGEKALIGFS TSFAEYILMDPSPEYAPIFGLMQEKIYISKIVVEFLQSNSDSTYEDLINKIETTVPPSGL NLNRFTEDSLLRHAQFVVEQVESYDEAGDSDEQPIFLTPCMRDLIKLAGVTLGQRRAQAR RQTIRHSTREKDRGPTKATTTKLVYQIFDTFFAEQIEKDDREDKENAFKRRRCGVCEVCQ QPECGKCKACKDMVKFGGSGRSKQACQERRCPNMAMKEADDDEEVDDNIPEMPSPKKMHQ GKKKKQNKNRISWVGEAVKTDGKKSYYKKVCIDAETLEVGDCVSVIPDDSSKPLYLARVT ALWEDSSNGQMFHAHWFCAGTDTVLGATSDPLELFLVDECEDMQLSYIHSKVKVIYKAPS ENWAMEGGMDPESLLEGDDGKTYFYQLWYDQDYARFESPPKTQPTEDNKFKFCVSCARLA EMRQKEIPRVLEQLEDLDSRVLYYSATKNGILYRVGDGVYLPPEAFTFNIKLSSPVKRPR KEPVDEDLYPEHYRKYSDYIKGSNLDAPEPYRIGRIKEIFCPKKSNGRPNETDIKIRVNK FYRPENTHKSTPASYHADINLLYWSDEEAVVDFKAVQGRCTVEYGEDLPECVQVYSMGGP NRFYFLEAYNAKSKSFEDPPNHARSPGNKGKGKGKGKGKPKSQACEPSEPEIEIKLPKLR TLDVFSGCGGLSEGFHQAGISDTLWAIEMWDPAAQAFRLNNPGSTVFTEDCNILLKLVMA GETTNSRGQRLPQKGDVEMLCGGPPCQGFSGMNRFNSRTYSKFKNSLVVSFLSYCDYYRP RFFLLENVRNFVSFKRSMVLKLTLRCLVRMGYQCTFGVLQAGQYGVAQTRRRAIILAAAP GEKLPLFPEPLHVFAPRACQLSVVVDDKKFVSNITRLSSGPFRTITVRDTMSDLPEVRNG ASALEISYNGEPQSWFQRQLRGAQYQPILRDHICKDMSALVAARMRHIPLAPGSDWRDLP NIEVRLSDGTMARKLRYTHHDRKNGRSSSGALRGVCSCVEAGKACDPAARQFNTLIPWCL PHTGNRHNHWAGLYGRLEWDGFFSTTVTNPEPMGKQGRVLHPEQHRVVSVRECARSQGFP DTYRLFGNILDKHRQVGNAVPPPLAKAIGLEIKLCMLAKARESASAKIKEEEAAKD Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Methylates CpG residues. Preferentially methylates hemimethylated DNA. Associates with DNA replication sites in S phase maintaining the methylation pattern in the newly synthesized strand, that is essential for epigenetic inheritance. Associates with chromatin during G2 and M phases to maintain DNA methylation independently of replication. It is responsible for maintaining methylation patterns established in development. DNA methylation is coordinated with methylation of histones. Mediates transcriptional repression by direct binding to HDAC2. In association with DNMT3B and via the recruitment of CTCFL/BORIS, involved in activation of BAG1 gene expression by modulating dimethylation of promoter histone H3 at H3K4 and H3K9. Probably forms a corepressor complex required for activated KRAS-mediated promoter hypermethylation and transcriptional silencing of tumor suppressor genes (TSGs) or other tumor-related genes in colorectal cancer (CRC) cells. Also required to maintain a transcriptionally repressive state of genes in undifferentiated embryonic stem cells (ESCs). Associates at promoter regions of tumor suppressor genes (TSGs) leading to their gene silencing. Promotes tumor growth.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
3 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Ovarian tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.91E-04 Fold-change: 2.12E-01 Z-score: 6.38E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Owing to the aberrant methylation engendered by DNMT1 over-expression, miR-30a-5p, and miR-30c-5p levels dropped significantly in cisplatin-resistant ovarian cancer (OC) cells. On the contrary, miR-30a/c-5p inhibited Snail and DNMT1 directly. Hence, a feedback loop between DNMT1 and miR-30a/c-5p could be a potential signature for addressing EMT and cisplatin resistance in OC. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Resistant Drug | Cisplatin | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | Owing to the aberrant methylation engendered by DNMT1 over-expression, miR-30a-5p, and miR-30c-5p levels dropped significantly in cisplatin-resistant ovarian cancer (OC) cells. On the contrary, miR-30a/c-5p inhibited Snail and DNMT1 directly. Hence, a feedback loop between DNMT1 and miR-30a/c-5p could be a potential signature for addressing EMT and cisplatin resistance in OC. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [2] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| A2780 cells | Ovary | Homo sapiens (Human) | CVCL_0134 | |
| HO8910 cells | Ovary | Homo sapiens (Human) | CVCL_6868 | |
| CAOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0201 | |
| ES2 cells | Ovary | Homo sapiens (Human) | CVCL_AX39 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay; Flow cytometric analysis | |||
| Mechanism Description | miR30a/c-5p in turn directly inhibited DNMT1 as well as Snail. Forced expression of miR30a/c-5p or knocking down of DNMT1 and Snail promoted cisplatin susceptibility and partially reversed epithelial-mesenchymal transition (EMT) in CP70 cells. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [3] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell viability | Inhibition | hsa05200 | ||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| A2780 cells | Ovary | Homo sapiens (Human) | CVCL_0134 | |
| A2780CP cells | Ovary | Homo sapiens (Human) | CVCL_0135 | |
| HIOSE-80 cells | Ovary | Homo sapiens (Human) | CVCL_E274 | |
| OV119 cells | Ovary | Homo sapiens (Human) | N.A. | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | miR-200b- and miR-200c-mediated downregulation of DNMTs may improve chemotherapeutic efficacy by increasing the sensitivity of cancer cells. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [4] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Inhibition | hsa04670 | |
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometry assay | |||
| Mechanism Description | The data showed a down-regulated of miR-148b expression and evaluated methyltransferases (DNMTs) expression in cisplatin-resisted human non-small cell lung cancer (NSCLC) cell line-A549/DDP and SPC-A1/DDP compared with their parental A549 and SPC-A1 cell line. In transfection experiments, miR-148b mimics reduced the DNMT1 expression, as well as (+) the sensitivity of cells to cisplatin and cisplatin-induced apoptosis in A549/DDP or SPC-A1/DDP cells. While miR-148b inhibitor increased DNMT1 expression, as well as attenuated the sensitivity of cells to cisplatin in A549 and SPC-A1 cells. miR-148b was showed to exert negative effect on DNMT1 expression by targeting its 3'UTR in A549/DDP and A549 cells. Importantly, silenced DNMT1 increases cisplatin sensitivity of A549/DDP cells and over-expressed DNMT1 reverses pro-apoptosis effect of miR-148b mimic. | |||
| Disease Class: Ovarian cancer [ICD-11: 2C73.0] | [5] | |||
| Sensitive Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Chemoresistance | Inhibition | hsa05207 | ||
| In Vitro Model | SkOV3 cells | Ovary | Homo sapiens (Human) | CVCL_0532 |
| A2780 cells | Ovary | Homo sapiens (Human) | CVCL_0134 | |
| A2780/DDP cells | Ovary | Homo sapiens (Human) | CVCL_D619 | |
| In Vivo Model | CD-1/CD-1 nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-152 and miR-185 were involved in cisplatin resistance, miR-152 and miR-185 increased cisplatin sensitivity mainly through the direct downregulation of DNMT1. DNMT1 is the most abundant DNA methyltransferase in mammalian cells and the key enzyme for the maintenance of hemimethylated DNA during DNA replication and de novo methylation during somatic cell development and differentiation. DNMT1 expression is also upregulated in many malignancies. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Osteosarcoma [ICD-11: 2B51.0] | [6] | |||
| Resistant Disease | Osteosarcoma [ICD-11: 2B51.0] | |||
| Resistant Drug | Decitabine | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Inhibition | hsa04210 | |
| Cell viability | Activation | hsa05200 | ||
| HOTAIR-miR126-DNMT1-CDkN2A axis | Regulation | N.A. | ||
| In Vitro Model | MG63 cells | Bone marrow | Homo sapiens (Human) | CVCL_0426 |
| SAOS-2 cells | Bone marrow | Homo sapiens (Human) | CVCL_0548 | |
| U2OS cells | Bone | Homo sapiens (Human) | CVCL_0042 | |
| HOS cells | Bone | Homo sapiens (Human) | CVCL_0312 | |
| 143B cells | Bone | Homo sapiens (Human) | CVCL_2270 | |
| NHOst cells | Bone | Homo sapiens (Human) | N.A. | |
| HFob 1.19 | Bone | Homo sapiens (Human) | CVCL_3708 | |
| Experiment for Molecule Alteration |
RT-qPCR; Western blot analysis; ChIP assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | HOTAIR activates the expression of DNMT1 through repressing miR126, which is the negative regulator of DNMT1. HOTAIR depletion increases the sensibility of OS cells to DNMT1 inhibitor through regulating the viability and apoptosis of OS cells via HOTAIR-miR126-DNMT1-CDkN2A axis. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Malaria [ICD-11: 1F45.0] | [7] | |||
| Sensitive Disease | Malaria [ICD-11: 1F45.0] | |||
| Sensitive Drug | Procainamide | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | The fusion of the histone deacetylase inhibitor SAHA and the DNMT inhibitor procainamide into a single compound, Proca-SAHA, resulted in an original family of antimalarials that is highly potent and selective for Plasmodium. Importantly, Proca-SAHA derivatives are highly active against multiple P. falciparum isolates from Cambodia, the epicenter of malaria drug-resistance, which makes them excellent drug candidates. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.56E-81; Fold-change: 7.26E-01; Z-score: 2.22E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 5.94E-48; Fold-change: 5.74E-01; Z-score: 2.09E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
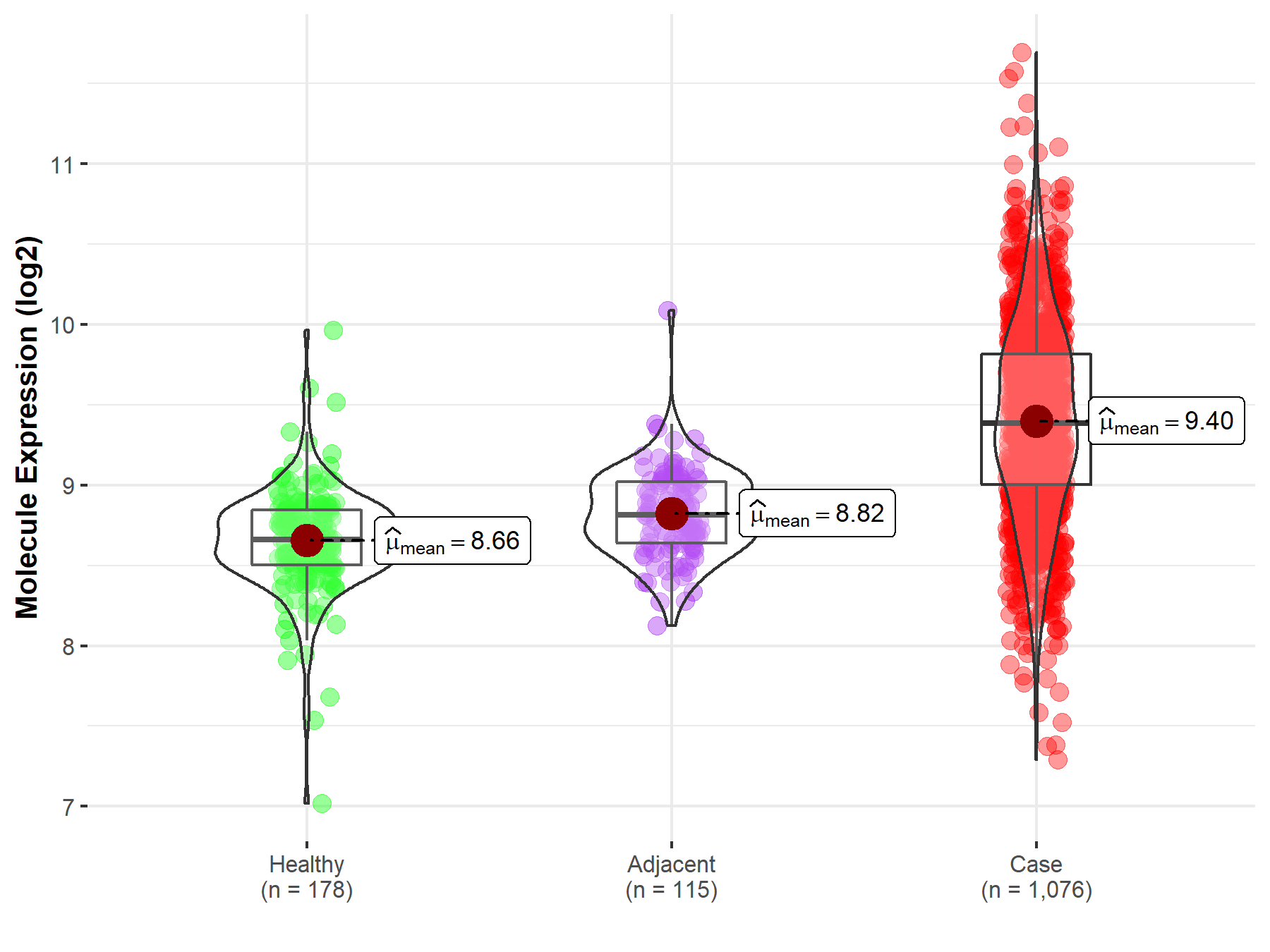
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Ovary | |
| The Specified Disease | Ovarian cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.91E-04; Fold-change: 1.36E+00; Z-score: 2.28E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 8.54E-01; Fold-change: 3.51E-01; Z-score: 3.11E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
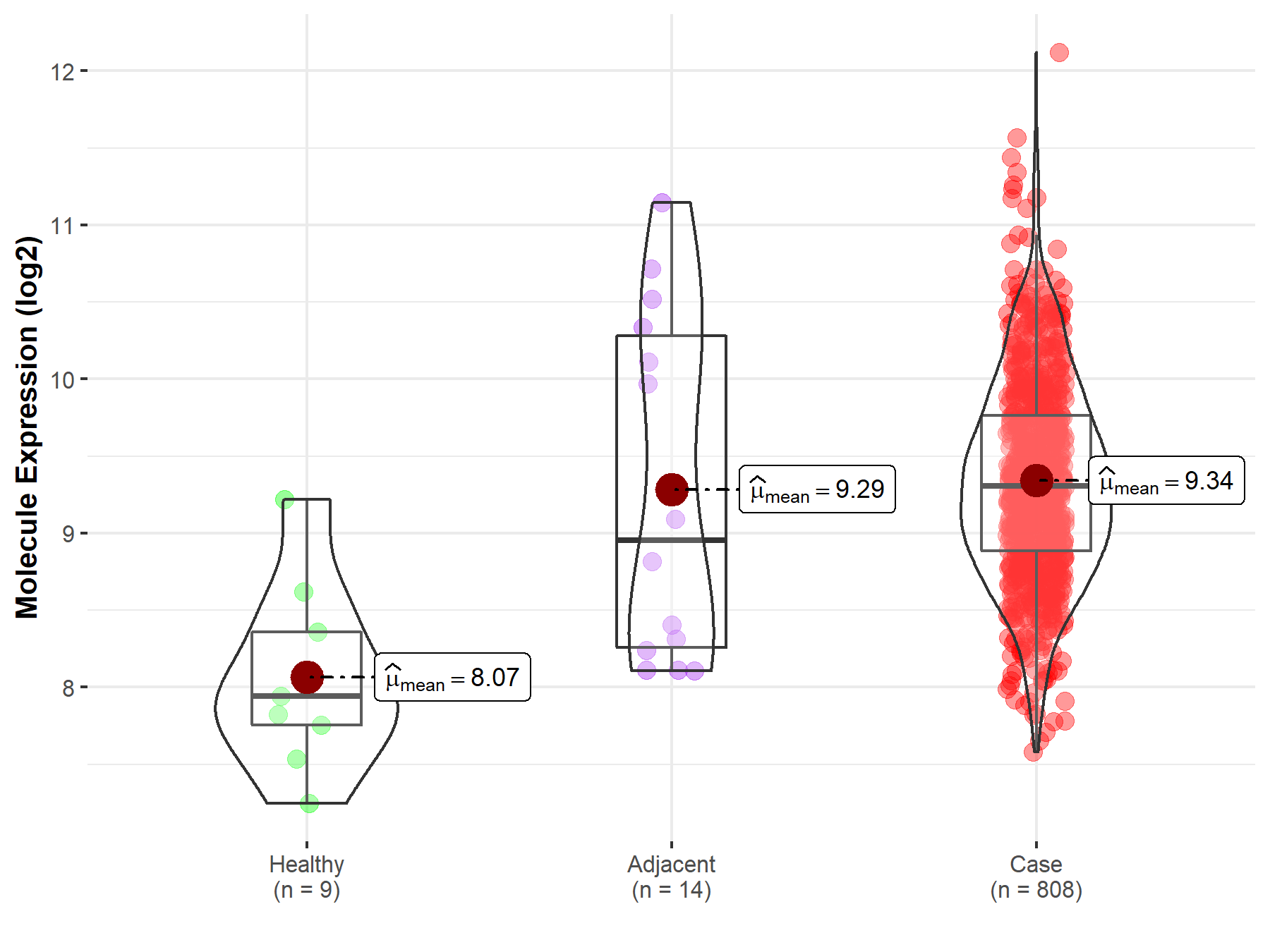
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

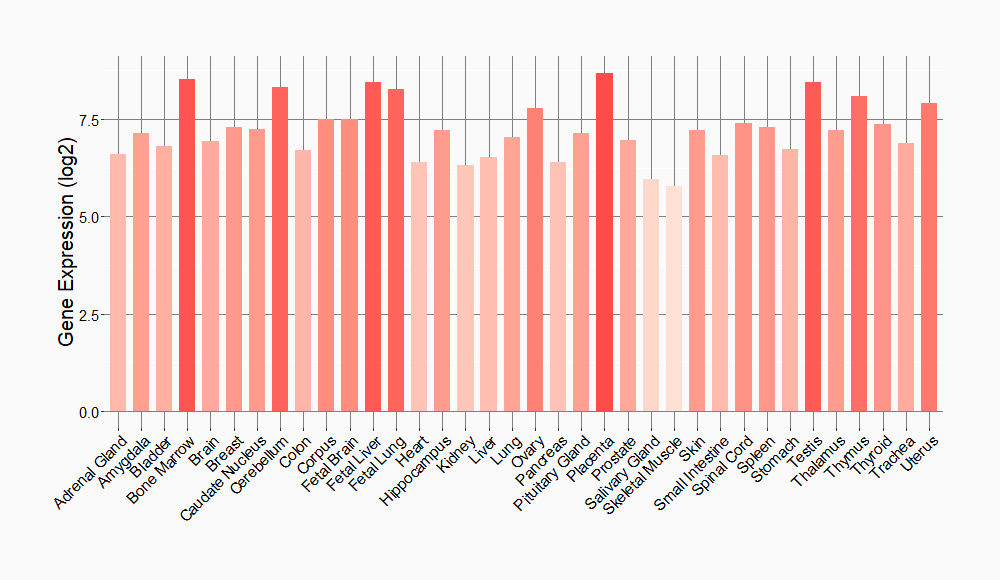
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
