Drug Information
Drug (ID: DG00799) and It's Reported Resistant Information
| Name |
Procainamide
|
||||
|---|---|---|---|---|---|
| Synonyms |
PROCAINAMIDE; 51-06-9; Novocainamide; Biocoryl; Novocamid; 4-Amino-N-[2-(diethylamino)ethyl]benzamide; Procaine amide; Pronestyl; Novocainamid; Procamide; 4-amino-N-(2-diethylaminoethyl)benzamide; Novocaine amide; Procainamida; Procainamidum; Procan; Procapan (free base); p-Aminobenzoic diethylaminoethylamide; p-Amino-N-(2-diethylaminoethyl)benzamide; Pronestyl-Sr; 4-Amino-N-(2-(diethylamino)ethyl)benzamide; UNII-L39WTC366D; NSC 27461; CHEBI:8428; 2-Diethylaminoethylamid kyseliny p-aminobenzoove; Benzamide, 4-amino-N-(2-(diethylamino)ethyl)-; Benzamide, 4-amino-N-[2-(diethylamino)ethyl]-; L39WTC366D; Benzamide, p-amino-N-(2-(diethylamino)ethyl)-; Benzamide, p-amino-N-[2-(diethylamino)ethyl]-; NSC-27461; NCGC00015859-07; Rhythmin; DSSTox_CID_3512; Procanbid; SP 100; SP 100 (pharmaceutical); DSSTox_RID_77059; DSSTox_GSID_23512; Procainamide [INN:BAN]; Procainamidum [INN-Latin]; Procainamida [INN-Spanish]; CAS-51-06-9; Procainamide (INN); HSDB 3170; CAS-614-39-1; EINECS 200-078-8; BRN 2214285; 2-Diethylaminoethylamid kyseliny p-aminobenzoove [Czech]; Spectrum_000836; Maybridge1_004389; Prestwick0_000337; Prestwick1_000337; Prestwick2_000337; Prestwick3_000337; Spectrum2_001295; Spectrum3_000555; Spectrum4_000487; Spectrum5_000986; Lopac-P-9391; CHEMBL640; Epitope ID:135397; Sp 100 (pharmaceutical); Cambridge id 5144127; Lopac0_000995; SCHEMBL15914; BSPBio_000373; BSPBio_001463; BSPBio_002229; CBDivE_003757; KBioGR_000183; KBioGR_000973; KBioSS_000183; KBioSS_001316; 4-14-00-01154 (Beilstein Handbook Reference); cid_66068; BIDD:GT0579; DivK1c_000931; SPBio_001329; SPBio_002294; WLN: ZR DVM2N2&2; BPBio1_000411; GTPL4811; DTXSID7023512; BDBM39344; HMS553P13; HY-A0084A; KBio1_000931; KBio2_000183; KBio2_001316; KBio2_002751; KBio2_003884; KBio2_005319; KBio2_006452; KBio3_000365; KBio3_000366; KBio3_001729; NINDS_000931; Bio1_000391; Bio1_000880; Bio1_001369; Bio2_000183; Bio2_000663; HMS1361J05; HMS1791J05; HMS1989J05; HMS2089E13; HMS3402J05; NSC27461; ZINC1530756; Tox21_110246; 7077AB; MFCD00066880; STK367963; AKOS000271131; Tox21_110246_1; 4-Amino-N-(diethylaminoethyl)benzamide; CCG-205075; CS-W009100; DB01035; FS-5697; MCULE-2863739556; SDCCGSBI-0050968.P005; IDI1_000931; IDI1_033933; SMP1_000055; NCGC00015859-01; NCGC00015859-02; NCGC00015859-03; NCGC00015859-04; NCGC00015859-05; NCGC00015859-06; NCGC00015859-08; NCGC00015859-09; NCGC00015859-10; NCGC00015859-11; NCGC00015859-14; NCGC00015859-16; NCGC00015859-18; NCGC00015859-23; NCGC00024323-03; NCGC00024323-04; NCGC00024323-05; NCGC00024323-06; N-(2-Diethylaminoethyl) 4-aminobenzamide; 4-amino-N-(2-diethylaminoethyl) benzamide; SBI-0050968.P004; AB00053530; BB 0216450; N1-[2-(diethylamino)ethyl]-4-aminobenzamide; 4-{N-[2-(diethylamino)ethyl]carbamoyl}aniline; 4-Amino-N-[2-(diethylamino)ethyl]benzamide #; C07401; C75392; D08421; AB00053530-13; AB00053530-15; AB00053530_16; 051P069; L001052; Q417597; BRD-K75089421-001-02-5; BRD-K75089421-003-04-7; BRD-K75089421-003-05-4; BRD-K75089421-003-15-3; Benzamide, 4-amino-N-(2-(diethylamino)ethyl)- (9CI); F2173-1035; 4-azanyl-N-[2-(diethylamino)ethyl]benzamide;hydrochloride
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
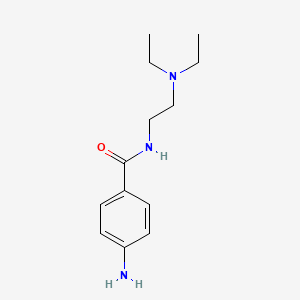
|
||||
| Target | Voltage-gated sodium channel (Nav) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C13H21N3O
|
||||
| IsoSMILES |
CCN(CC)CCNC(=O)C1=CC=C(C=C1)N
|
||||
| InChI |
1S/C13H21N3O/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17)
|
||||
| InChIKey |
REQCZEXYDRLIBE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: DNA (cytosine-5)-methyltransferase 1 (DNMT1) | [1] | |||
| Sensitive Disease | Malaria [ICD-11: 1F45.0] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | The fusion of the histone deacetylase inhibitor SAHA and the DNMT inhibitor procainamide into a single compound, Proca-SAHA, resulted in an original family of antimalarials that is highly potent and selective for Plasmodium. Importantly, Proca-SAHA derivatives are highly active against multiple P. falciparum isolates from Cambodia, the epicenter of malaria drug-resistance, which makes them excellent drug candidates. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
