Drug Information
Drug (ID: DG01818) and It's Reported Resistant Information
| Name |
Protirelin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Protirelin; Thyroliberin; 24305-27-9; Lopremone; Thyrotropin-releasing hormone; Thyrotropin releasing hormone; Rifathyroin; Synthetic TRH; Thypinone; TSH-releasing factor; TSH-releasing hormone; Thyrotropin-releasing factor; Thyrotropic-releasing factor; Thyrotropic releasing hormone; 5-Oxo-L-prolyl-L-histidyl-L-prolinamide; TRH; Abbott 38579; Thyroid releasing hormone; Thyrotropin-ReleasingHormone; Synthetic tsh-releasing factor; Synthetic tsh-releasing hormone; ABBOTT-38579; L-Pyroglutamyl-L-histidyl-L-prolineamide; UNII-5Y5F15120W; C16H22N6O4; Protirelin tartrate; FDA 1725; Protirelina; Ro 8-6270/9; L-Prolinamide, 5-oxo-L-prolyl-L-histidyl-; 11096-37-0; 117217-40-0; CHEBI:35940; 24305-27-9 (free base); L-Pyroglutamyl-L-histidyl-L-prolinamide; 5Y5F15120W; Synthetic thyrotropin-releasing hormone; (2S)-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide; NCGC00160616-01; L-Pyroglutamyl-L-histidinyl-L-prolinamide; A-38579; (S)-N-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl)-5-oxopyrrolidine-2-carboxamide; THYREL TRH; Protireline; Protirelinum; TRF; Protireline [INN-French]; Protirelinum [INN-Latin]; Protirelina [INN-Spanish]; Thyrothropin relasing hormone; Thyrefact; CCRIS 2593; trh-sr; Synthetic TRF; Relefact TRH; Relefact-TRH; NSC-760113; Protirelin [USAN:INN:BAN:JAN]; Stimu-TSH; Thyrotropin-releasing factor (pig); EINECS 246-143-4; PR 546; PR-546; Protirelin, synthetic; pGlu-His-Pro-NH2; Relefact TRH (TN); HOLO-TRANSFERRIN; PyroGlu-His-prolinamide; TSH-RF; 5-oxo-pro-his-pro-nh2; DSSTox_CID_3533; CHEMBL1472; DSSTox_RID_77067; DSSTox_GSID_23533; SCHEMBL33419; Thyrotrophin releasing hormone; [3H]-TRH; GTPL2139; GTPL3836; pyroglutamyl-histidyl-prolinamide; DTXSID0023533; Protirelin (JP17/USAN/INN); SCHEMBL19825647; [3H]TRH (human, mouse, rat); [3H]-thyrotropin-releasing hormone; BCP12450; HY-P0002; Thyrotropic hormone-releasing factor; ZINC4096261; Synthetic thyrotopin releasing factor; Thyrotropic hormone-releasing hormone; Tox21_111939; BDBM50072394; s4680; Synthetic thyrotropin-releasing factor; Thyrotrophic hormone releasing hormone; AKOS015994636; CCG-268193; DB09421; HS-2023; NSC 760113; L-Pyroglutamyl-L-histidyl-L-proline amide; CAS-24305-27-9; Thyroid-stimulating hormone-releasing factor; C03958; C74244; D00176; Prolinamide, 5-oxo-L-prolyl-L-histidyl-, L-; (Pyro)-L-glutamic acid-L-histidine-L-proline-NH2; 305T279; A858244; Q24726011; Thyrotropin releasing hormone, >=98% (HPLC), powder; Prolinamide, 1-[N-(5-oxo-L-prolyl)-L-histidyl]-, L-; Protirelin, European Pharmacopoeia (EP) Reference Standard; Thyrotropin releasing hormone, powder, gamma-irradiated, cell culture tested; Thyroliberin;Lopremone;Synthetic TRH;Thyrotropin-releasing hormone; Rifathyroin; (2S)-N-[(1S)-2-[(2S)-2-carbamoylpyrrolidino]-1-(1H-imidazol-5-ylmethyl)-2-keto-ethyl]-5-keto-pyrrolidine-2-carboxamide; (2S)-N-[(2S)-1-[(2S)-2-aminocarbonylpyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxidanylidene-propan-2-yl]-5-oxidanylidene-pyrrolidine-2-carboxamide; (2S)-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide; (2S)-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-3-(3H-imidazol-4-yl)-1-oxopropan-2-yl]-5-oxopyrrolidine-2-carboxamide; (S)-N-((S)-1-((S)-2-carbamoylpyrrolidin-1-yl)-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl)-5-oxopyrrolidine-2-carboxamide; 1-(3-(1H-Imidazol-5-yl)-2-([(5-oxo-2-pyrrolidinyl)carbonyl]amino)propanoyl)-2-pyrrolidinecarboxamide #; 1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyrrolylcarboxamido)propanoyl]tetrahydro-1H-2-pyrrolecarboxamide; 2-Pyrrolidinecarboxamide, N-[1-[(2-carbamoyl-1-pyrrolidinyl)carbonyl]-2-imidazol-4-ylethyl]-5-oxo-,; 2-Pyrrolidinecarboxamide, N-[1-[(2-Carbamoyl-1-pyrrolidinyl)carbonyl]-2-imidazol-4-ylethyl]-5-oxo-, (S,S,S)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
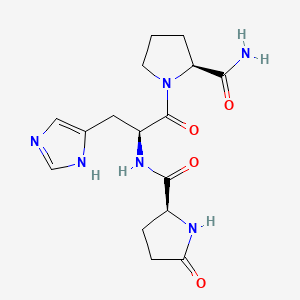
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Thyrotropin-releasing hormone receptor (TRHR) | TRFR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
C1C[C@H](N(C1)C(=O)[C@H](CC2=CN=CN2)NC(=O)[C@@H]3CCC(=O)N3)C(=O)N
|
||||
| InChI |
InChI=1S/C16H22N6O4/c17-14(24)12-2-1-5-22(12)16(26)11(6-9-7-18-8-19-9)21-15(25)10-3-4-13(23)20-10/h7-8,10-12H,1-6H2,(H2,17,24)(H,18,19)(H,20,23)(H,21,25)/t10-,11-,12-/m0/s1
|
||||
| InChIKey |
XNSAINXGIQZQOO-SRVKXCTJSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-13: Digestive system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Peptidylprolyl isomerase F (cyclophilin F) (Ppif) | [1] | |||
| Resistant Disease | Oxidative stress-related liver diseases [ICD-11: DB90-DB9Z] | |||
| Molecule Alteration | Up-regulation | Expression |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | Tshr -/- mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Microarray assay; qRT-PCR; ELISA assay; Western bloting analysis | |||
| Mechanism Description | TSH stimulates hepatic CypD acetylation through the LncRNA-AK044604/SIRT1/SIRT3 signaling pathway, indicating an essential role for TSH in mitochondrial stress in the liver. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
