Drug Information
Drug (ID: DG01789) and It's Reported Resistant Information
| Name |
Rotenone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Rotenone; 83-79-4; Dactinol; Paraderil; Barbasco; (-)-Rotenone; Tubatoxin; Derrin; (-)-cis-Rotenone; Rotocide; Derris; Rotenon; Cube root; Nicouline; Noxfish; Ronone; Canex; Deril; Liquid Derris; Cube-Pulver; Dri-Kil; Rotessenol; Noxfire; Rotefive; Rotefour; Rotenox; Cubor; Extrax; Haiari; Mexide; Cube extract; Derris root; Powder and root; Chem-Mite; Pro-nox fish; Fish-Tox; Cenol garden dust; Curex flea duster; Prenfish; Tubotoxin; Gerane; Prentox; Ro-Ko; Synpren; Derris (insecticide); Chem fish; Pb-nox; Rotenox 5EC; 5'-beta-Rotenone; Green Cross Warble Powder; Chem-Fish Synergized; Prentox Synpren-Fish; Caswell No. 725; Rotenone, dehydro; Foliafume E.C.; ENT 133; Rotenone, commercial; CCRIS 895; HSDB 1762; NCI-C55210; 5'beta-Rotenone; EPA Pesticide Chemical Code 071003; UNII-03L9OT429T; AI3-00133; NSC26258; Rotenona; Derris resins; MLS000738056; CHEMBL429023; CHEBI:28201; 03L9OT429T; Rotacide E.C.; CUBE; (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-2-alpha-isopropenyl-8,9-dimethoxy-; (12aS,6aS,2R)-8,9-dimethoxy-2-(1-methylvinyl)-1,2-dihydrochromano[3,4-b]furano [2,3-h]chroman-6-one; (2R,6aS,12aS)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one; (2R-(2alpha,6aalpha,12aalpha))-1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)(1)benzopyrano(3,4-b)furo(2,3-h)benzopyran-6(6aH)-one; 1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-(1)benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(6aH)-one; 1,2,12,12aalpha-Tetrahydro-2a-isopropenyl-8,9-dimethoxy(1)benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(6aH)-one; NCGC00017358-05; Nekoe; DSSTox_CID_1248; DSSTox_RID_76038; DSSTox_GSID_21248; (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, (2R-(2alpha,6aalpha,12aalpha))-; (1S,6R,13S)-16,17-dimethoxy-6-prop-1-en-2-yl-2,7,20-trioxapentacyclo[11.8.0.03,11.04,8.014,19]henicosa-3(11),4(8),9,14,16,18-hexaen-12-one; (2R,6aS,12aS)-1,2,6,6a,12,12a-hexahydro-2-isopropenyl-8,9-dimethoxychromeno[3,4-b]furo[2,3-h]chromen-6-one; (2R,6aS,12aS)-8,9-Dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydrofuro[2',3':7,8][1]benzopyrano[2,3-c][1]benzopyran-6(6aH)-one; [2R-(2alpha,6aalpha,12aalpha)]-1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6aH)-one; Rotenona [Spanish]; Rotenone [BSI:ISO]; Protax; MFCD09025614; 5'.beta.-Rotenone; CAS-83-79-4; (1)Benzopyrano(3,4-b)furo(2,3-h)(1)benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, (2R,6aS,12aS)-; (2R,6aS,12aS)-1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one; (2R,6aS,12aS)-1,2,6,6a,12,12a- hexahydro-2-isopropenyl-8,9- dimethoxychromeno[3,4-b] furo(2,3-h)chromen-6-one; (2R,6aS,12aS)-1,2,6,6a,12,12a-hexahydro-2-isopropenyl-8,9-dimethoxychromeno(3,4-b)furo(2,3-h)chromen-6-one; (2R,6aS,12aS)-2-isopropenyl-8,9-dimethoxy-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one; [1]Benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, (2R,6aS,12aS)-; SR-01000076110; EINECS 201-501-9; NSC 26258; Rotenoid; Roteonone; WLN: T G5 D6 B666 CV HO MO POT&TT&J IY1&U1 SO1 TO1; 5''beta-rotenone; 5'b-Rotenone; Rotocide E.C.; (1)Benzopyrano[3,3-h](1)benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-2-.alpha.-iospropenyl-8,9-dimethoxy-; [1]Benzopyrano[3,3-h][1]benzopyran-6(6a.alpha.H)-one, 1,2,12,12a.alpha.-tetrahydro-2.alpha.-isopropenyl-8,9-dimethoxy-; [1]Benzopyrano[3,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, [2R-(2.alpha.,6a.alpha.,12a.alpha.)]-; [1]Benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, [2R-(2alpha,6aalpha,12aalpha)]-; Derris, JMAF; Prestwick_542; Rotenone (7CI); Rotenone, >=95%; Spectrum_000065; Spectrum2_000457; Spectrum3_000158; Spectrum4_001638; Spectrum5_000455; Lopac0_001112; SCHEMBL42253; BSPBio_001896; isopropenyl(dimethoxy)[ ]one; KBioGR_002075; KBioSS_000465; SPECTRUM200013; (2R-(6aalpha,12aalpha)-1,2-Dihydro-2-isopropenyl-8,9-dimethoxychromano(3,4-b)furo(2,3-h)chroman-6-on; DivK1c_000947; SPBio_000534; DTXSID6021248; HMS502P09; KBio1_000947; KBio2_000465; KBio2_003033; KBio2_005601; KBio3_001116; inhibits NADH2 oxidation to NAD; NSC8505; NINDS_000947; HMS3263O06; 12679-58-2; BCP07278; HY-B1756; NSC-8505; TNP00301; ZINC3860715; Tox21_110819; Tox21_201904; Tox21_300695; Tox21_501112; 2901AC; BDBM50135527; CCG-39886; LMPK12060007; NSC-26258; AKOS004910398; Tox21_110819_1; CS-6067; DB11457; LP01112; MCULE-7287067191; MCULE-7415617623; SDCCGMLS-0066415.P001; SDCCGSBI-0051081.P003; IDI1_000947; Rotenone 100 microg/mL in Acetonitrile; NCGC00017358-01; NCGC00017358-02; NCGC00017358-03; NCGC00017358-04; NCGC00017358-06; NCGC00017358-07; NCGC00017358-08; NCGC00017358-09; NCGC00017358-10; NCGC00017358-11; NCGC00017358-14; NCGC00017358-15; NCGC00017358-22; NCGC00094382-01; NCGC00094382-02; NCGC00094382-03; NCGC00094382-04; NCGC00094382-05; NCGC00254603-01; NCGC00259453-01; NCGC00261797-01; AC-31290; AS-10183; NCI60_002093; SMR000393729; EU-0101112; R0090; Rotenone, PESTANAL(R), analytical standard; C07593; R 8875; 599T610; Q412388; SR-01000076110-2; SR-01000076110-5; SR-01000076110-6; W-104134; BRD-K08316444-001-01-9; BRD-K08316444-001-05-0; (2R-(6aalpha,12aalpha)-1,2-Dihydro-2-isopropenyl-8,9-dimethoxychromano(3,4-b)furo(2,3-h)chroman-6-one; [1]Benzopyrano[3,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-2-.alpha.-isopropenyl-8,9-dimethoxy-; [1]Benzopyrano[3,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-2-isopropenyl-8,9-dimethoxy-; [1]Benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aalphaH)-one, 1,2,12,12aalpha-tetrahydro-2alpha-isopropenyl-8,9-dimethoxy- (8CI); [1]Benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-, (2R,6aS,12aS)- (9CI); 1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one, 9CI; Tubatoxin 1,2,12,12a,-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-h][1]-benzopyran-6(6aH)-one
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
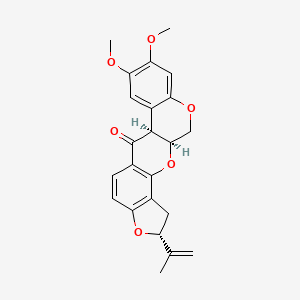
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
3
|
||||
| IsoSMILES |
CC(=C)[C@H]1CC2=C(O1)C=CC3=C2O[C@@H]4COC5=CC(=C(C=C5[C@@H]4C3=O)OC)OC
|
||||
| InChI |
InChI=1S/C23H22O6/c1-11(2)16-8-14-15(28-16)6-5-12-22(24)21-13-7-18(25-3)19(26-4)9-17(13)27-10-20(21)29-23(12)14/h5-7,9,16,20-21H,1,8,10H2,2-4H3/t16-,20-,21+/m1/s1
|
||||
| InChIKey |
JUVIOZPCNVVQFO-HBGVWJBISA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Small nucleolar RNA host gene 14 (SNHG14) | [1] | |||
| Resistant Disease | Parkinson disease [ICD-11: 8A00.0] | |||
| Molecule Alteration | Up-regulation | Interaction |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | SNHG14/miR-133b/alpha -syn signaling pathway | Inhibition | hsa04933 | |
| In Vitro Model | MN9D cells | N.A. | Mus musculus (Mouse) | CVCL_M067 |
| In Vivo Model | C57BL/6 mice xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Knockdown assay | |||
| Experiment for Drug Resistance |
Motor function assessment assay; CCK8 assay; ROS staining and detection assay | |||
| Mechanism Description | Silence of SNHG14 mitigates dopaminergic neuron injury by down-regulating alpha-syn via targeting miR-133b, which contributes to improving PD. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
