Drug Information
Drug (ID: DG01788) and It's Reported Resistant Information
| Name |
Medroxyprogesterone acetate
|
||||
|---|---|---|---|---|---|
| Synonyms |
Medroxyprogesterone acetate; Medroxyprogesterone 17-acetate; 71-58-9; Provera; Metigestrona; Farlutin; Gestapuran; Perlutex; Veramix; Methylacetoxyprogesterone; Medroxyacetate progesterone; Depo-Provera; Depcorlutin; Deporone; Lutopolar; Nadigest; Prodasone; Progestalfa; Progevera; Proverone; Repromix; Sirprogen; Supprestral; Lutoral; Nidaxin; Oragest; Depo-Promone; Promone-E; 6-alpha-Methyl-17-alpha-acetoxyprogesterone; Medroxyprogesteroneacetate; NSC-26386; 6-alpha-Methyl-17-alpha-hydroxyprogesterone acetate; 17alpha-Hydroxy-6alpha-methylprogesterone acetate; 6alpha-Methyl-17alpha-hydroxyprogesterone acetate; Amen; 6alpha-Methyl-4-pregnene-3,20-dion-17alpha-ol acetate; Depot medroxyprogesterone acetate; Depot-medroxyprogesterone acetate; UNII-C2QI4IOI2G; DP150; Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6alpha)-; 17-Acetoxy-6alpha-methylprogesterone; Aragest; Clinovir; Ralovera; 6alpha-Methyl-17-acetoxy progesterone; Hysron; Provera dosepak; Depo-clinovir; Depo-Ralovera; (6alpha)-6-methyl-3,20-dioxopregn-4-en-17-yl acetate; U 8839; C2QI4IOI2G; Medroxyprogesterone (acetate); Medroxyprogesterone-17-acetate; MLS000069442; (6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-dione; CHEBI:6716; ONCO-Provera; NSC-21171; Medrosterona; Clinofem; Cykrina; Depocon; Indivina; Mepastat; Meprate; Repromap; SMR000059125; Sumiferm; Suprestral; Veraplex; Dugen; Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-, (6a)-; Depo-progestin; Depo-Prodasone; Depo-Progevera; Perlutex Leo; DSSTox_CID_5527; Depo-Map; Aragest 5; Med-Pro; MPA-beta; DSSTox_RID_77819; MPA Hexal; MPA-Noury; DSSTox_GSID_25527; [(6S,8R,9S,10R,13S,14S,17R)-17-acetyl-6,10,13-trimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-yl] acetate; MAP (steroid); 17alpha-Acetoxy-6alpha-methylprogesterone; Depomedroxyprogesterone acetate; MPA GYM; Depo-Medroxyprogesterone acetate; Depo-subq provera; CAS-71-58-9; CCRIS 371; 17.alpha.-Acetoxy-6.alpha.-methylprogesterone; EINECS 200-757-9; 6alpha-Methyl-17-acetoxyprogesterone; NSC 21171; Depo-Provera Contraceptive; 17-Acetoxy-6-alpha-methylprogesterone; (6.alpha.-Pregn-4-ene-3, 17-(acetyloxy)-6-methyl-; 17.alpha.-Hydroxy-6-.alpha.-methylprogesterone acetate; Pregn-4-ene-3, 17-hydroxy-6.alpha.-methyl-, acetate; 6-alpha-Methyl-17-acetoxy progesterone; BRN 2066112; Pregn-4-ene-3, 17-(acetyloxy)-6-methyl-, (6.alpha.)-; AI3-60127; 17-Acetoxy-6.alpha.-methylprogesterone; 6.alpha.-Methyl-17-acetoxyprogesterone; Farlutal inyectable; NCGC00094713-01; 17alpha-Hydroxy-6-alpha-methylprogesterone acetate; Progesterone, 17-hydroxy-6alpha-methyl-, acetate; 17-alpha-Hydroxy-6-alpha-methylprogesterone acetate; Provera (TN); Depo-provera (TN); 6alpha-Methyl-17alpha-acetoxypregn-4-ene-3,20-dione; Medroxyprogesterone acetate [USP:JAN]; 17-alpha-Acetoxy-6-alpha-methylpregn-4-ene-3,20-dione; 17alpha-Acetoxy-6-alpha-methylpregn-4-ene-3,20-dione; 6-alpha-Methyl-17-alpha-acetoxypregn-4-ene-3,20-dione; Progesterone, 17-alpha-hydroxy-6-alpha-methyl-, acetate; Medroxyprogesterone acetate [Progestins]; 6-alpha-Methyl-4-pregnene-3,20-dion-17-alpha-ol acetate; 6.alpha.-Methyl-17.alpha.-acetoxyprogesterone; (6-alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-dione; 17alpha-Hydroxy-6alpha-methylpregn-4-ene-3,20-dione acetate; 6alpha-Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-; CPD000653524; 17-alpha-Hydroxy-6-alpha-methylpregn-4-ene-3,20-dione acetate; Medroxyprogesteroni acetas; Opera_ID_1110; medroxyprogesterone-acetate; 17.alpha.-Hydroxy-6.alpha.-methylprogesterone acetate; 6.alpha.-Methyl-17.alpha.-hydroxyprogesterone acetate; Medroxiprogesterone Acetate; CHEMBL717; SCHEMBL4276; 6.alpha.-Methyl-17.alpha.-acetoxypregn-4-ene-3,20-dione; 17.alpha.-Acetoxy-6-.alpha.-methylpregn-4-ene-3,20-dione; 4-08-00-02212 (Beilstein Handbook Reference); 6.alpha.-Methyl-4-pregnene-3,20-dion-17.alpha.-ol acetate; MLS001148217; MLS002207115; 17.alpha.-Hydroxy-6.alpha.-methylpregn-4-ene-3,20-dione acetate; Depo-subq provera 104 (TN); DTXSID0025527; G03AC06; HMS2233O07; HMS2235E05; HMS3259O14; HMS3884B10; (6-alpha)-Pregn-4-ene-3,20-dione, 17-(acetyloxy)-6-methyl-; (6S,8R,9S,10R,13S,14S,17R)-17-Acetyl-6,10,13-trimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl acetate; CBP-1011; HY-B0469; NSC21171; NSC26386; ZINC5029557; Tox21_111319; Tox21_200141; BDBM50067678; s2567; AKOS015894870; Tox21_111319_1; AC-2174; CCG-264965; DB00603; NC00574; Medroxyprogesterone acetate (JP17/USP); MRF-0000023; NCGC00022037-03; NCGC00022037-04; NCGC00022037-05; NCGC00022037-07; NCGC00257695-01; NCGC00263480-01; AS-13981; I028; SMR000653524; TV-46046; C08150; C76275; D00951; AB00384270-14; AB00384270_15; Medroxyprogesterone 17-acetate, >=97% (HPLC); 010M483; Q2823834; (6 )-6-Methyl-3,20-dioxopregn-4-en-17-yl acetate; 6.alpha.-Pregn-4-ene-3, 17-(acetyloxy)-6-methyl-; Medroxyprogesterone-17-acetate 100 microg/mL in Acetonitrile; Medroxyprogesterone 17-acetate, VETRANAL(TM), analytical standard; Medroxyprogesterone acetate, European Pharmacopoeia (EP) Reference Standard; Pregn-4-ene-3,20-dione, 17-hydroxy-6alpha-methyl-, acetate (8CI); Medroxyprogesterone Acetate, Pharmaceutical Secondary Standard; Certified Reference Material; Medroxyprogesterone acetate, United States Pharmacopeia (USP) Reference Standard; Medroxyprogesterone acetate for peak identification, European Pharmacopoeia (EP) Reference Standard; Medroxyprogesterone acetate for system suitability, European Pharmacopoeia (EP) Reference Standard; Medroxyprogesterone acetate, for performance test, European Pharmacopoeia (EP) Reference Standard
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
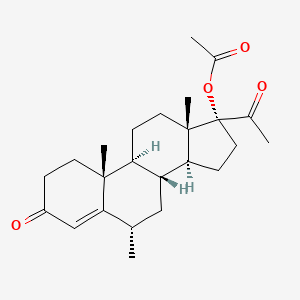
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
3
|
||||
| IsoSMILES |
C[C@H]1C[C@@H]2[C@H](CC[C@]3([C@H]2CC[C@@]3(C(=O)C)OC(=O)C)C)[C@@]4(C1=CC(=O)CC4)C
|
||||
| InChI |
InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1
|
||||
| InChIKey |
PSGAAPLEWMOORI-PEINSRQWSA-N
|
||||
| PubChem CID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Long non-protein coding RNA (LNC-CETP-3) | [1] | |||
| Resistant Disease | Endometrial adenocarcinoma [ICD-11: 2C76.0] | |||
| Molecule Alteration | Up-regulation | Expression |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ishikawa cells | Endometrium | Homo sapiens (Human) | CVCL_2529 |
| KLE cells | Ovary | Homo sapiens (Human) | CVCL_1329 | |
| Experiment for Molecule Alteration |
Microarray assay; qRT-PCR | |||
| Mechanism Description | Medroxyprogesterone acetate causes the alterations of endoplasmic reticulum related mRNAs and LncRNAs in endometrial cancer cells. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
