Drug Information
Drug (ID: DG01775) and It's Reported Resistant Information
| Name |
Melatonin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Melatonin; 73-31-4; Melatonine; N-Acetyl-5-methoxytryptamine; N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide; Circadin; 5-Methoxy-N-acetyltryptamine; N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide; Melatol; Melovine; Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-; N-[2-(5-methoxyindol-3-yl)ethyl]acetamide; N-(2-(5-Methoxyindol-3-yl)ethyl)acetamide; UNII-JL5DK93RCL; N-acetyl-5-methoxy-tryptamine; NSC 113928; 8041-44-9; CHEMBL45; MFCD00005655; JL5DK93RCL; Acetamide, N-(2-(5-methoxyindol-3-yl)ethyl)-; 3-(n-acetyl-2-aminoethyl)-5-methoxyindole; Melatonex; CHEBI:16796; Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]-; NSC-56423; NSC113928; NSC-113928; CAS-73-31-4; NCGC00015680-11; DSSTox_CID_2421; DSSTox_RID_76585; DSSTox_GSID_22421; Melapure; Posidorm; Primex; WLN: T56 BMJ D2MV1 GO1; [3H]melatonin; Melatonina (TN); [3H]-melatonin; ML1; SMR000326666; CCRIS 3472; N-(2-(5-methoxyindol-3-yl)ethyl)-Acetamide; N-[2-(5-methoxyindol-3-yl)ethyl]-Acetamide; N-[2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide; SR-01000075559; EINECS 200-797-7; BRN 0205542; [3H]MLT; Acetamide, N-(2-(5-methoxy-1H-indol-3-yl)ethyl)- (9CI); Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]- (9CI); Melatonine;; Guna-dermo; HSDB 7509; Melatobel (TN); Melatonin (JAN); TNP00300; Prestwick_312; N-[2-(5-Methoxy-1H-indol-3-yl)-ethyl]-acetamide; Spectrum_000185; Guna-dermo (Salt/Mix); Primex (8CI,9CI); Prestwick0_000458; Prestwick1_000458; Prestwick2_000458; Prestwick3_000458; Spectrum2_001344; Spectrum3_001393; Spectrum4_000066; Spectrum5_001745; Lopac-M-5250; M1105; ChemDiv2_003916; M 5250; M-1200; M-1250; Melatonin, >=99.5%; Lopac0_000787; Oprea1_104553; Oprea1_814234; SCHEMBL19018; BSPBio_000536; BSPBio_003006; GTPL224; KBioGR_000591; KBioSS_000665; 5-22-12-00042 (Beilstein Handbook Reference); Acetamide, {N-[2-(5-methoxyindol-3-yl)ethyl]-}; MLS000859594; MLS001055382; MLS001240204; 5-methoxy n-acetyl-tryptamine; BIDD:ER0618; DivK1c_000353; SPECTRUM1500690; SPBio_001527; SPBio_002475; Melatonin (synth.) ultra-pure; BDBM9019; BPBio1_000590; GTPL1357; Acetamide, {N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-}; DTXSID1022421; HMS501B15; KBio1_000353; KBio2_000665; KBio2_003233; KBio2_005801; KBio3_002226; ZINC57060; BCI-049; Melatonin 1.0 mg/ml in Methanol; NINDS_000353; 3-N-Acetyl-5-methoxyl tryptamine; HMS1380B22; HMS1569K18; HMS1921E04; HMS2089F09; HMS2096K18; HMS2233D23; HMS3262M16; HMS3370J20; HMS3413P14; HMS3654A22; HMS3677P14; HMS3713K18; HMS3884M05; Melatonin (synth.) standard-grade; ACT03490; AMY33320; BCP28154; HY-B0075; NSC56423; Tox21_110195; Tox21_201527; Tox21_302926; Tox21_500787; CCG-38837; HSCI1_000400; Melatonin, powder, >=98% (TLC); STK386880; AKOS000276269; Tox21_110195_1; CS-1769; DB01065; J5.258B; KS-1454; LP00787; MCULE-1497884152; SDCCGMLS-0065812.P001; SDCCGMLS-0065812.P002; SDCCGSBI-0050765.P003; IDI1_000353; IDI1_002631; SMP2_000309; N-acetyl-5-methoxy-tryptamine Melatonine; NCGC00015680-01; NCGC00015680-02; NCGC00015680-03; NCGC00015680-04; NCGC00015680-05; NCGC00015680-06; NCGC00015680-07; NCGC00015680-08; NCGC00015680-09; NCGC00015680-10; NCGC00015680-12; NCGC00015680-13; NCGC00015680-14; NCGC00015680-15; NCGC00015680-16; NCGC00015680-18; NCGC00015680-35; NCGC00090727-01; NCGC00090727-02; NCGC00090727-03; NCGC00090727-04; NCGC00090727-05; NCGC00090727-06; NCGC00090727-07; NCGC00090727-08; NCGC00090727-09; NCGC00256404-01; NCGC00259077-01; NCGC00261472-01; AC-10019; BA164660; NCI60_004378; SY051401; AB00053279; EU-0100787; FT-0628191; FT-0658928; FT-0670984; S1204; SW196607-4; C01598; D08170; J10164; AB00053279-10; AB00053279_12; {N-[2-(5-methoxyindol-3-yl)ethyl]-} Acetamide; 005M655; A929721; L001261; N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-Acetamide; Q180912; SR-01000075559-1; SR-01000075559-6; SR-01000075559-7; SR-01000075559-8; {N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-} Acetamide; BRD-K97530723-001-07-6; BRD-K97530723-001-11-8; F1929-1777; Melatonin, British Pharmacopoeia (BP) Reference Standard; 0E2B08C1-B325-45B1-8939-6F9081EFDFA4; Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]- (6CI,8CI); Melatonin, United States Pharmacopeia (USP) Reference Standard; Melatonin, Pharmaceutical Secondary Standard; Certified Reference Material; Melatonin solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
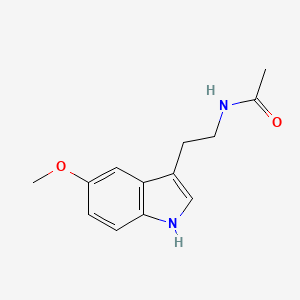
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Melatonin receptor type 1A (MTNR1A) | MTR1A_HUMAN | [1] | ||
| Quinone reductase 2 (NQO2) | NQO2_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
4
|
||||
| IsoSMILES |
CC(=O)NCCC1=CNC2=C1C=C(C=C2)OC
|
||||
| InChI |
InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16)
|
||||
| InChIKey |
DRLFMBDRBRZALE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Long non-protein coding RNA (LNC010561) | [1] | |||
| Resistant Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Down-regulation | Interaction |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vivo Model | BALB/c nude mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Knockdown assay; Microarray assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Knockdown of FKBP3 and lnc010561 inhibited breast cancer proliferation and invasion, and induced apoptosis. | |||
ICD-04: Immune system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Monocyte chemotactic and activating factor (CCL2) | [2] | |||
| Resistant Disease | Autoimmune disease [ICD-11: 4A4Z.0] | |||
| Molecule Alteration | Down-regulation | Expression |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Knockdown assay | |||
| Mechanism Description | The conclusion on a partial mediation by SIRT1 is supported by repeatedly observed inhibitions of melatonin effects by sirtuin inhibitors or knockdown. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
