Drug Information
Drug (ID: DG01667) and It's Reported Resistant Information
| Name |
SB590885
|
||||
|---|---|---|---|---|---|
| Synonyms |
SB590885; 405554-55-4; SB-590885; 5-(2-(4-(2-(dimethylamino)ethoxy)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)-2,3-dihydro-1H-inden-1-one oxime; SB 590885; (NE)-N-[5-[2-[4-[2-(dimethylamino)ethoxy]phenyl]-5-pyridin-4-yl-1H-imidazol-4-yl]-2,3-dihydroinden-1-ylidene]hydroxylamine; (E)-5-(2-(4-(2-(dimethylamino)ethoxy)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)-2,3-dihydro-1H-inden-1-one oxime; (E)-SB-590885; (Z)-SB-590885; SCHEMBL131578; CHEMBL477989; SCHEMBL12518520; SCHEMBL16111665; SCHEMBL17378611; EX-A612; CHEBI:131881; BCPP000070; 1H-Inden-1-one, 5-[2-[4-[2-(dimethylamino)ethoxy]phenyl]-5-(4-pyridinyl)-1H-imidazol-4-yl]-2,3-dihydro-, oxime; 5-[2-[4-[2-(Dimethylamino)ethoxy]phenyl]-5-(4-pyridinyl)-1H-imidazol-4-yl]-2,3-dihydro-1H-inden-1-one oxime; AMY20674; BDBM50457452; NSC754362; NSC756456; s2220; SB-590885 (RAF); ZINC100061199; CCG-264947; NSC-754362; NSC-756456; (E)--(2-(dimethylamino)ethoxy)phenyl)-5-(pyridin-4-yl)-1H-imidazol-4-yl)-2,3-dihydro-1H-inden-1-one oxime; (E)-SB590885; AS-16232; (E)-SB 590885; A25514; J-501805; BRD-K78809024-001-05-7; Q27120375; Q27225284; (1E)-N-{5-[2-{4-[2-(dimethylamino)ethoxy]phenyl}-4-(pyridin-4-yl)-1H-imidazol-5-yl]-2,3-dihydro-1H-inden-1-ylidene}hydroxylamine; 5-[2-[4-[2-(Dimethylamino)ethoxy]phenyl]-5-(4-pyridinyl)-1H-imidazol-4-yl]-2,3-dihydro-1H-inden-1-one oxime;SB-590885
Click to Show/Hide
|
||||
| Structure |
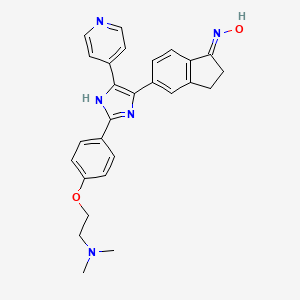
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
7
|
||||
| IsoSMILES |
CN(C)CCOC1=CC=C(C=C1)C2=NC(=C(N2)C3=CC=NC=C3)C4=CC5=C(C=C4)/C(=N/O)/CC5
|
||||
| InChI |
InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,33H,6,10,15-16H2,1-2H3,(H,29,30)/b31-24+
|
||||
| InChIKey |
MLSAQOINCGAULQ-QFMPWRQOSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| In Vivo Model | Nude mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
BRAF kinase assay | ||||||||||||
| Experiment for Drug Resistance |
Flow cytometry assay | ||||||||||||
| Mechanism Description | The missense mutation p.V600E (c.1799T>A) in gene BRAF cause the sensitivity of SB590885 by aberration of the drug's therapeutic target | ||||||||||||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Collagen triple helix repeat-containing protein 1 (CTHRC1) | [1] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Mutations | Arg622Ile |
||
| Experiment for Molecule Alteration |
qRT-PCR; Immune cells infiltrating assay | |||
| Experiment for Drug Resistance |
Immunohistochemistry assay; Pearson correlation testing | |||
| Mechanism Description | Colon cancer, thyroid cancer, and melanoma are common malignant tumors that seriously threaten human health globally. The B-Raf proto-oncogene, serine/threonine kinase (BRAF)(V600E) mutation is an important driver gene mutation in these cancer types. In this study, we identified that collagen triple helix repeat containing 1 (CTHRC1) expression was associated with the BRAF(V600E) mutation in colon cancer, thyroid cancer, and melanoma. A high level of CTHRC1 was correlated with decreased sensitivity to antitumor drugs (vemurafenib, PLX-4720, dabrafenib, and SB-590885) targeting the BRAF(V600E) mutation. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | SW1736 cells | Thyroid | Homo sapiens (Human) | CVCL_3883 | |||||||||
| 8505C cells | Thyroid | Homo sapiens (Human) | CVCL_1054 | ||||||||||
| Hth104 cells | Thyroid gland | Homo sapiens (Human) | CVCL_A427 | ||||||||||
| In Vivo Model | Mouse xenograft model | Mus musculus | |||||||||||
| Mechanism Description | The missense mutation p.V600E (c.1799T>A) in gene BRAF cause the sensitivity of SB590885 by aberration of the drug's therapeutic target | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
