Drug Information
Drug (ID: DG01644) and It's Reported Resistant Information
| Name |
PLX8394
|
||||
|---|---|---|---|---|---|
| Synonyms |
PLX8394; 1393466-87-9; PLX-8394; UNII-J2L7Z273SG; J2L7Z273SG; PLX 8394; (3R)-N-[3-[5-(2-cyclopropylpyrimidin-5-yl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl]-3-fluoropyrrolidine-1-sulfonamide; (R)-N-(3-(5-(2-cyclopropylpyrimidin-5-yl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)-3-fluoropyrrolidine-1-sulfonamide; GTPL9131; CHEMBL4303729; SCHEMBL15666953; BDBM317826; BCP19619; EX-A1461; PLX 8394;PLX8394; NSC797932; NSC801007; s7965; ZINC144705377; CS-5123; NSC-797932; NSC-801007; US9624213, Compound P-0338; NCGC00483921-01; BP168493; BS-15485; HY-18972; A16840; D83660; A900333; Q27088419; 1-Pyrrolidinesulfonamide, N-(3-((5-(2-cyclopropyl-5-pyrimidinyl)-1H-pyrrolo(2,3-b)pyridin-3-yl)carbonyl)-2,4-difluorophenyl)-3-fluoro-, (3R)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
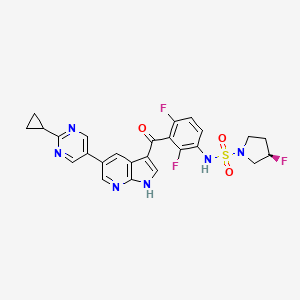
|
||||
| Target | Fms-like tyrosine kinase 3 (FLT-3) | FLT3_HUMAN | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
7
|
||||
| IsoSMILES |
C1CN(C[C@@H]1F)S(=O)(=O)NC2=C(C(=C(C=C2)F)C(=O)C3=CNC4=C3C=C(C=N4)C5=CN=C(N=C5)C6CC6)F
|
||||
| InChI |
InChI=1S/C25H21F3N6O3S/c26-16-5-6-34(12-16)38(36,37)33-20-4-3-19(27)21(22(20)28)23(35)18-11-32-25-17(18)7-14(8-31-25)15-9-29-24(30-10-15)13-1-2-13/h3-4,7-11,13,16,33H,1-2,5-6,12H2,(H,31,32)/t16-/m1/s1
|
||||
| InChIKey |
YYACLQUDUDXAPA-MRXNPFEDSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Synonymous | p.K601K (c.1803A>G) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Synonymous | p.G464G (c.1392A>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G469R (c.1405G>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G469A (c.1406G>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G469V (c.1406G>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Synonymous | p.L597L (c.1791A>T) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | PRT cells | Brain | Homo sapiens (Human) | CVCL_7207 | |||||||||
| 1205Lu cells | Skin | Homo sapiens (Human) | CVCL_5239 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTT assay; AlamarBlue assay; Colony growth assay | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Missense mutation | p.G469A (c.1406G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HCC364 cells | Lung | Homo sapiens (Human) | CVCL_5134 |
| H2405 cells | Lung | Homo sapiens (Human) | CVCL_1551 | |
| H2087 cells | Lymph node | Homo sapiens (Human) | CVCL_1524 | |
| H1755 cells | Liver | Homo sapiens (Human) | CVCL_1492 | |
| H1666 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1485 | |
| H1437 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1472 | |
| H1395 cells | Lung | Homo sapiens (Human) | CVCL_1467 | |
| CAL-12T cells | Lung | Homo sapiens (Human) | CVCL_1105 | |
| In Vivo Model | SCID beige mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Promega assay | |||
| Mechanism Description | PLX8394 was effective against treatment-naive BRAF-mutant LAs and those with acquired vemurafenib resistance caused by an alternatively spliced, truncated BRAFV600E that promotes vemurafenib-insensitive MAPK pathway signaling. Acquired PLX8394 resistance occurs via EGFR-mediated RAS-mTOR signaling and is prevented by upfront combination therapy with PLX8394 and either an EGFR or mTOR inhibitor. | |||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | |||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Molecule Alteration | Missense mutation | p.G466V (c.1397G>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HCC364 cells | Lung | Homo sapiens (Human) | CVCL_5134 |
| H2405 cells | Lung | Homo sapiens (Human) | CVCL_1551 | |
| H2087 cells | Lymph node | Homo sapiens (Human) | CVCL_1524 | |
| H1755 cells | Liver | Homo sapiens (Human) | CVCL_1492 | |
| H1666 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1485 | |
| H1437 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1472 | |
| H1395 cells | Lung | Homo sapiens (Human) | CVCL_1467 | |
| CAL-12T cells | Lung | Homo sapiens (Human) | CVCL_1105 | |
| In Vivo Model | SCID beige mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Promega assay | |||
| Mechanism Description | PLX8394 was effective against treatment-naive BRAF-mutant LAs and those with acquired vemurafenib resistance caused by an alternatively spliced, truncated BRAFV600E that promotes vemurafenib-insensitive MAPK pathway signaling. Acquired PLX8394 resistance occurs via EGFR-mediated RAS-mTOR signaling and is prevented by upfront combination therapy with PLX8394 and either an EGFR or mTOR inhibitor. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
