Drug Information
Drug (ID: DG01613) and It's Reported Resistant Information
| Name |
Tazemetostat
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tazemetostat; 1403254-99-8; EPZ-6438; EPZ6438; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; E7438; UNII-Q40W93WPE1; EPZ 6438; E-7438; Tazemetostat (EPZ-6438); Q40W93WPE1; MFCD24849415; N-[(4,6-dimethyl-2-oxo-1H-pyridin-3-yl)methyl]-3-[ethyl(oxan-4-yl)amino]-2-methyl-5-[4-(morpholin-4-ylmethyl)phenyl]benzamide; Tazemetostat [INN]; Tazverik; Tazemetostat [USAN]; Tazemetostat (USAN/INN); Tazemetostat [USAN:INN]; E 7438; Tazemetostat(EPZ-6438); GTPL7011; CHEMBL3414621; SCHEMBL13276848; SCHEMBL19325409; SCHEMBL22210809; EX-A509; HMS3653O07; HMS3747A09; AOB87748; BCP07409; EPZ-7438; 2399AH; NSC777109; NSC791066; s7128; AKOS026750211; ZINC100285161; CCG-264672; CS-1758; DB12887; NSC-777109; NSC-791066; SB22955; NCGC00381562-01; NCGC00381562-10; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-; AC-30931; AS-55991; DA-34776; HY-13803; N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(oxan-4-yl)amino)-4-methyl-4'-((morpholin-4-yl)methyl)(1,1'-biphenyl)-3-carboxamide; QC-11696; SY040988; FT-0700193; SW220030-1; A12712; D11444; J-690163; J-690164; Q27088941; EPZ-6438; EPZ 6438; E7438; Tazemetostat; E-7438; E 7438; (R,Z)-1-(1-(1-(ethylsulfonyl)piperidin-4-yl)ethyl)-N-((2-hydroxy-4-methoxy-6-methylpyridin-3-yl)methyl)-2-methyl-1H-indole-3-carbimi dic acid; 5-(Ethyl(tetrahydro-2H-pyran-4-yl)amino)-N-((2-hydroxy-4,6-dimethylpyridin-3-yl)methyl)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl) methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)biphenyl-3-carboxamide; N-[(4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-5-[ethyl(tetrahydropyran-4-yl)amino]-4-methyl-4 inverted exclamation mark -(morpholinomethyl)biphenyl-3-carboxamide
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
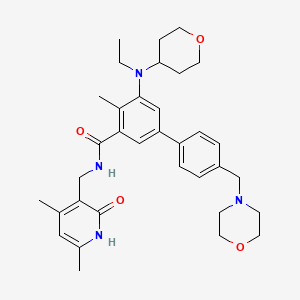
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[3]
|
||||
| Target | Fibroblast growth factor receptor (FGFR) | NOUNIPROTAC | [3] | ||
| Fibroblast growth factor receptor 1 (FGFR1) | FGFR1_HUMAN | [3] | |||
| Fibroblast growth factor receptor 2 (FGFR2) | FGFR2_HUMAN | [3] | |||
| Fibroblast growth factor receptor 3 (FGFR3) | FGFR3_HUMAN | [3] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
9
|
||||
| IsoSMILES |
CCN(C1CCOCC1)C2=CC(=CC(=C2C)C(=O)NCC3=C(C=C(NC3=O)C)C)C4=CC=C(C=C4)CN5CCOCC5
|
||||
| InChI |
InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40)
|
||||
| InChIKey |
NSQSAUGJQHDYNO-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Histone-lysine N-methyltransferase EZH2 (EZH2) | [3] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.Y111D (c.331T>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 |
| Pfeiffer cells | Pleural effusion | Homo sapiens (Human) | CVCL_3326 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Histone-lysine N-methyltransferase EZH2 (EZH2) | [2] | |||
| Resistant Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Complex-indel | p.T678_R679delinsKK (c.2032_2037delinsAAGAAG) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | RN2c cells | Blood | Mus musculus (Mouse) | N.A. |
| RN2 cells | Blood | Mus musculus (Mouse) | N.A. | |
| Plat-E cells | Fetal kidney | Homo sapiens (Human) | CVCL_B488 | |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Histone-lysine N-methyltransferase EZH2 (EZH2) | [1] | |||
| Sensitive Disease | Lymphoma [ICD-11: 2A90- 2A85] | |||
| Molecule Alteration | Missense mutation | p.Y646F (c.1937A>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | CRL-2959 cells | Peritoneal effusion | Homo sapiens (Human) | CVCL_2206 |
| CRL-2632 cells | Pleural effusion | Homo sapiens (Human) | CVCL_3326 | |
| CRL-2631 cells | Peripheral blood | Homo sapiens (Human) | CVCL_3611 | |
| CRL-2630 cells | Lymph node | Homo sapiens (Human) | CVCL_3302 | |
| CRL-2261 cells | Ascites | Homo sapiens (Human) | CVCL_1660 | |
| ACC-576 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1889 | |
| ACC-575 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1902 | |
| ACC-528 cells | Bone marrow | Homo sapiens (Human) | CVCL_1878 | |
| ACC-32 cells | Pleural effusion | Homo sapiens (Human) | CVCL_1325 | |
| In Vivo Model | Sprague-Dawley rat model | Rattus norvegicus | ||
| Experiment for Molecule Alteration |
ChIP-PCR | |||
| Experiment for Drug Resistance |
Cell Titer Glo assay; Tumor volume measurement assay; Immunohistochemistry assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
