Drug Information
Drug (ID: DG01605) and It's Reported Resistant Information
| Name |
SR-9009
|
||||
|---|---|---|---|---|---|
| Synonyms |
SR9009; 1379686-30-2; SR-9009; SR 9009; ethyl 3-[[(4-chlorophenyl)methyl-[(5-nitrothiophen-2-yl)methyl]amino]methyl]pyrrolidine-1-carboxylate; Stenabolic (SR9009); CHEMBL1961796; 1-Pyrrolidinecarboxylic acid, 3-((((4-chlorophenyl)methyl)((5-nitro-2-thienyl)methyl)amino)methyl)-, ethyl ester; Stenabolic; 1-pyrrolidinecarboxylic acid, 3-[[[(4-chlorophenyl)methyl][(5-nitro-2-thienyl)methyl]amino]methyl]-, ethyl ester; REV-ERB Agonist II; GTPL8901; EX-A726; BCP16215; BDBM50366238; MFCD29472236; NSC810521; s8692; AKOS027470307; CCG-269102; CS-4669; DB14013; NSC-810521; SB19006; NCGC00384202-01; AC-30219; AS-55859; HY-16989; A886340; J-690150; Q15410184; ethyl 3-(4-chlorobenzyl)((5-nitrothiophen-2-yl)methylaminomethyl)pyrrolidine-1-carboxylate; N'-[(1E)-1-(5-Chloro-2-hydroxyphenyl)ethylidene]-3-(4-morpholinylsulfonyl)benzohydrazide; ethyl 3-[[(4-chlorophenyl)methyl-[(5-nitro-2-thienyl)methyl]amino]methyl]pyrrolidine-1-carboxylate; Ethyl 3-[[[(4-chlorophenyl)methyl][(5-nitro-2-thienyl)methyl]amino]methyl]-1-pyrrolidinecarboxylate
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
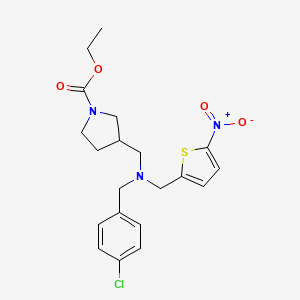
|
||||
| Target | ALK tyrosine kinase receptor (ALK) | ALK_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
8
|
||||
| IsoSMILES |
CCOC(=O)N1CCC(C1)CN(CC2=CC=C(C=C2)Cl)CC3=CC=C(S3)[N+](=O)[O-]
|
||||
| InChI |
InChI=1S/C20H24ClN3O4S/c1-2-28-20(25)23-10-9-16(13-23)12-22(11-15-3-5-17(21)6-4-15)14-18-7-8-19(29-18)24(26)27/h3-8,16H,2,9-14H2,1H3
|
||||
| InChIKey |
MMJJNHOIVCGAAP-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: GTPase Hras (HRAS) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G12V (c.35G>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.98 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.96 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
0
|
S
-
M
M
T
T
E
E
Y
Y
K
K
L
L
V
V
V
V
V
V
10
|
G
G
A
A
G
V
G
G
V
V
G
G
K
K
S
S
A
A
L
L
20
|
T
T
I
I
Q
Q
L
L
I
I
Q
Q
N
N
H
H
F
F
V
V
30
|
D
D
E
E
Y
Y
D
D
P
P
T
T
I
I
E
E
D
D
S
S
40
|
Y
Y
R
R
K
K
Q
Q
V
V
V
V
I
I
D
D
G
G
E
E
50
|
T
T
C
C
L
L
L
L
D
D
I
I
L
L
D
D
T
T
A
A
60
|
G
G
Q
Q
E
E
E
E
Y
Y
S
S
A
A
M
M
R
R
D
D
70
|
Q
Q
Y
Y
M
M
R
R
T
T
G
G
E
E
G
G
F
F
L
L
80
|
C
C
V
V
F
F
A
A
I
I
N
N
N
N
T
T
K
K
S
S
90
|
F
F
E
E
D
D
I
I
H
H
H
H
Y
Y
R
R
E
E
Q
Q
100
|
I
I
K
K
R
R
V
V
K
K
D
D
S
S
E
E
D
D
V
V
110
|
P
P
M
M
V
V
L
L
V
V
G
G
N
N
K
K
S
S
D
D
120
|
L
L
P
P
S
S
R
R
T
T
V
V
D
D
T
T
K
K
Q
Q
130
|
A
A
Q
Q
D
D
L
L
A
A
R
R
S
S
Y
Y
G
G
I
I
140
|
P
P
F
F
I
I
E
E
T
T
S
S
A
A
K
K
T
T
R
R
150
|
Q
Q
G
G
V
V
D
D
D
D
A
A
F
F
Y
Y
T
T
L
L
160
|
V
V
R
R
E
E
I
I
R
R
K
K
H
H
K
K
E
E
K
K
170
|
M
M
S
S
K
K
D
D
G
G
K
K
K
K
K
K
K
K
K
K
180
|
K
K
S
S
K
K
T
T
K
K
C
C
V
V
I
I
M
M
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | HCT116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | |||||||||
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | ||||||||||
| Sk-Mel28 cells | Skin | Homo sapiens (Human) | CVCL_0526 | ||||||||||
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | ||||||||||
| PANC-1 cells | Pancreas | Homo sapiens (Human) | CVCL_0480 | ||||||||||
| Jurkat cells | Pleural effusion | Homo sapiens (Human) | CVCL_0065 | ||||||||||
| MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 | ||||||||||
| WI38 cells | Fetal lung | Homo sapiens (Human) | CVCL_0579 | ||||||||||
| BJ-ELR cells | N.A. | Homo sapiens (Human) | N.A. | ||||||||||
| BJ cells | Peripheral blood | Homo sapiens (Human) | CVCL_E483 | ||||||||||
| Becker cells | N.A. | Homo sapiens (Human) | CVCL_1093 | ||||||||||
| In Vivo Model | NOD mouse PDX model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Immunofluorescence microscopy assay; qRT-PCR | ||||||||||||
| Mechanism Description | Pharmacological modulation of circadian regulators is an effective novel antitumor strategy, identifying the existence of a previously unknown class of anticancer agents with a wide therapeutic window. REV-ERB agonists are novel autophagy and de novo lipogenesis inhibitors with selective activity towards malignant and benign neoplasms. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
