Drug Information
Drug (ID: DG01588) and It's Reported Resistant Information
| Name |
BMS-911543
|
||||
|---|---|---|---|---|---|
| Synonyms |
BMS-911543; 1271022-90-2; BMS911543; BMS 911543; UNII-7N03P021J8; N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridine-7-carboxamide; 7N03P021J8; N,N-Dicyclopropyl-4-[(1,5-Dimethyl-1h-Pyrazol-3-Yl)amino]-6-Ethyl-1-Methyl-1,6-Dihydroimidazo[4,5-D]pyrrolo[2,3-B]pyridine-7-Carboxamide; N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo(4,5-d)pyrrolo(2,3b)pyridine-7-carboxamide; GTPL7954; SCHEMBL1512419; CHEMBL3545215; DTXSID00155403; HMS3747C07; AOB87390; BCP07258; EX-A1551; WAC02290; BDBM50122318; MFCD22200575; NSC766821; NSC799366; s7144; AKOS027251175; JAK2 INHIBITOR BMS-911543; ZINC100468481; CCG-269045; CS-3237; DB12591; NSC-766821; NSC-799366; SB16625; NCGC00345798-01; NCGC00345798-06; AC-29054; AS-17031; HY-15270; Imidazo[4,5-d]pyrrolo[2,3-b]pyridine-7-carboxamide, N,N-dicyclopropyl-4-[(1,5-dimethyl-1H-pyrazol-3-yl)amino]-6-ethyl-1,6-dihydro-1-methyl-; QC-11446; FT-0697510; A908362; Q27075379; 50V; N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo(4,5-d); N,N-dicyclopropyl-4-(1,5-dimethyl-1H-pyrazol-3-ylamino)-6-ethyl-1-methyl-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridine-7-carboxamide; N,N-dicyclopropyl-7-[(1,5-dimethylpyrazol-3-yl)amino]-10-ethyl-3-methyl-3,5,8,10-tetrazatricyclo[7.3.0.02,6]dodeca-1,4,6,8,11-pentaene-11-carboxamide
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
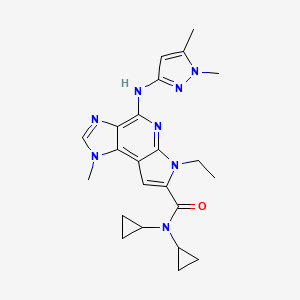
|
||||
| Target | Serine/threonine-protein kinase B-raf (BRAF) | BRAF_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
CCN1C(=CC2=C3C(=C(N=C21)NC4=NN(C(=C4)C)C)N=CN3C)C(=O)N(C5CC5)C6CC6
|
||||
| InChI |
InChI=1S/C23H28N8O/c1-5-30-17(23(32)31(14-6-7-14)15-8-9-15)11-16-20-19(24-12-28(20)3)21(26-22(16)30)25-18-10-13(2)29(4)27-18/h10-12,14-15H,5-9H2,1-4H3,(H,25,26,27)
|
||||
| InChIKey |
JCINBYQJBYJGDM-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Sensitive Disease | Myeloproliferative neoplasm [ICD-11: 2A22.0] | |||
| Molecule Alteration | Missense mutation | p.V617F (c.1849G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
[3H] thymidine incorporation assay | |||
| Mechanism Description | The missense mutation p.V617F (c.1849G>T) in gene JAK2 cause the sensitivity of BMS-911543 by aberration of the drug's therapeutic target | |||
|
|
||||
| Key Molecule: Thrombopoietin receptor (TPOR) | [1] | |||
| Sensitive Disease | Myeloproliferative neoplasm [ICD-11: 2A22.0] | |||
| Molecule Alteration | Missense mutation | p.W515L (c.1544G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
[3H] thymidine incorporation assay | |||
| Mechanism Description | The missense mutation p.W515L (c.1544G>T) in gene MPL cause the sensitivity of BMS-911543 by unusual activation of pro-survival pathway | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.V617F (c.1849G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
[3H] thymidine incorporation assay | |||
| Mechanism Description | The missense mutation p.V617F (c.1849G>T) in gene JAK2 cause the sensitivity of BMS-911543 by aberration of the drug's therapeutic target | |||
|
|
||||
| Key Molecule: Thrombopoietin receptor (TPOR) | [1] | |||
| Sensitive Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.W515L (c.1544G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
[3H] thymidine incorporation assay | |||
| Mechanism Description | The missense mutation p.W515L (c.1544G>T) in gene MPL cause the sensitivity of BMS-911543 by unusual activation of pro-survival pathway | |||
ICD-03: Blood/blood-forming organs diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Sensitive Disease | Essential thrombocythemia [ICD-11: 3B63.Z] | |||
| Molecule Alteration | Missense mutation | p.V617F (c.1849G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
[3H] thymidine incorporation assay | |||
| Mechanism Description | The missense mutation p.V617F (c.1849G>T) in gene JAK2 cause the sensitivity of BMS-911543 by aberration of the drug's therapeutic target | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
