Drug Information
Drug (ID: DG01571) and It's Reported Resistant Information
| Name |
Merestinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Merestinib; 1206799-15-6; LY2801653; LY-2801653; N-(3-fluoro-4-((1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl)oxy)phenyl)-1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxamide; UNII-5OGS5K699E; Merestinib (LY2801653); 5OGS5K699E; N-[3-fluoro-4-[1-methyl-6-(1H-pyrazol-4-yl)indazol-5-yl]oxyphenyl]-1-(4-fluorophenyl)-6-methyl-2-oxopyridine-3-carboxamide; N-(3-Fluoro-4-{[1-Methyl-6-(1h-Pyrazol-4-Yl)-1h-Indazol-5-Yl]oxy}phenyl)-1-(4-Fluorophenyl)-6-Methyl-2-Oxo-1,2-Dihydropyridine-3-Carboxamide; C30H22F2N6O3; N-(3-Fluoro-4-(1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yloxy)phenyl)-1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxamide; N-[3-Fluoro-4-[[1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl]oxy]phenyl]-1-(4-fluorophenyl)-1,2-dihydro-6-methyl-2-oxo-3-pyridinecarboxamide; Merestinib [USAN]; L1X; Merestinib (USAN/INN); SCHEMBL679002; LY2801653 (Merestinib); GTPL9841; CHEMBL3545307; DTXSID20659635; QCR-139; SYN1222; HMS3741E21; BCP06826; EX-A1264; 3797AH; BDBM50172078; NSC772197; NSC800788; s7014; ZINC95926668; AKOS027323283; CCG-270011; CS-1192; DB12381; NSC-772197; NSC-800788; SB16550; NCGC00378921-02; NCGC00378921-07; 3-Pyridinecarboxamide, N-(3-fluoro-4-((1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl)oxy)phenyl)-1-(4-fluorophenyl)-1,2-dihydro-6-methyl-2-oxo-; AS-16348; DA-21192; HY-15514; FT-0745076; LY 2801653; J3.563.073B; D11763; Q27262653
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
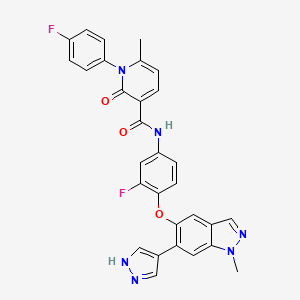
|
||||
| Target | Proto-oncogene c-Met (MET) | MET_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
CC1=CC=C(C(=O)N1C2=CC=C(C=C2)F)C(=O)NC3=CC(=C(C=C3)OC4=C(C=C5C(=C4)C=NN5C)C6=CNN=C6)F
|
||||
| InChI |
InChI=1S/C30H22F2N6O3/c1-17-3-9-23(30(40)38(17)22-7-4-20(31)5-8-22)29(39)36-21-6-10-27(25(32)12-21)41-28-11-18-16-35-37(2)26(18)13-24(28)19-14-33-34-15-19/h3-16H,1-2H3,(H,33,34)(H,36,39)
|
||||
| InChIKey |
QHADVLVFMKEIIP-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1010Y (c.3028G>T) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | ||||||||||
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | ||||||||||
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | ||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1003F (c.3008A>T) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |||||||||
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | ||||||||||
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | ||||||||||
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | ||||||||||
| Experiment for Molecule Alteration |
Direct sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CCK-8 assay | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [2] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228N (c.3682G>A) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ | |||||||||
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | ||||||||||
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | ||||||||||
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | ||||||||||
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | ||||||||||
| HCT-116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | ||||||||||
| Calu1 cells | Lung | Homo sapiens (Human) | CVCL_0608 | ||||||||||
| DU145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 | ||||||||||
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | ||||||||||
| U87-MG cells | Brain | Homo sapiens (Human) | CVCL_0022 | ||||||||||
| U118 MG cells | Brain | Homo sapiens (Human) | CVCL_0633 | ||||||||||
| TT cells | Thyroid gland | Homo sapiens (Human) | CVCL_1774 | ||||||||||
| H441 cells | Pericardial effusion | Homo sapiens (Human) | CVCL_1561 | ||||||||||
| H1993 cells | Lymph node | Homo sapiens (Human) | CVCL_1512 | ||||||||||
| In Vivo Model | Athymic nude mouse and CD-1 nude mouse xenograft model | Mus musculus | |||||||||||
| Mechanism Description | The missense mutation p.D1228N (c.3682G>A) in gene MET cause the sensitivity of Merestinib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [2] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y1230C (c.3689A>G) |
|||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HT29 Cells | Colon | Homo sapiens (Human) | CVCL_A8EZ | |||||||||
| A375 cells | Skin | Homo sapiens (Human) | CVCL_0132 | ||||||||||
| H460 cells | Lung | Homo sapiens (Human) | CVCL_0459 | ||||||||||
| T47D cells | Breast | Homo sapiens (Human) | CVCL_0553 | ||||||||||
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | ||||||||||
| HCT-116 cells | Colon | Homo sapiens (Human) | CVCL_0291 | ||||||||||
| Calu1 cells | Lung | Homo sapiens (Human) | CVCL_0608 | ||||||||||
| DU145 cells | Prostate | Homo sapiens (Human) | CVCL_0105 | ||||||||||
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | ||||||||||
| U87-MG cells | Brain | Homo sapiens (Human) | CVCL_0022 | ||||||||||
| U118 MG cells | Brain | Homo sapiens (Human) | CVCL_0633 | ||||||||||
| TT cells | Thyroid gland | Homo sapiens (Human) | CVCL_1774 | ||||||||||
| H441 cells | Pericardial effusion | Homo sapiens (Human) | CVCL_1561 | ||||||||||
| H1993 cells | Lymph node | Homo sapiens (Human) | CVCL_1512 | ||||||||||
| In Vivo Model | Athymic nude mouse and CD-1 nude mouse xenograft model | Mus musculus | |||||||||||
| Mechanism Description | The missense mutation p.Y1230C (c.3689A>G) in gene MET cause the sensitivity of Merestinib by aberration of the drug's therapeutic target | ||||||||||||
|
|
|||||||||||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D1228V (c.3683A>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.71 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.67 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
G
-
D
1040
|
-
S
-
D
-
I
-
S
-
S
-
P
-
L
-
L
-
Q
N
N
1050
|
T
T
V
V
H
H
I
I
D
D
L
L
S
S
A
A
L
L
N
N
1060
|
P
P
E
E
L
L
V
V
Q
Q
A
A
V
V
Q
Q
H
H
V
V
1070
|
V
V
I
I
G
G
P
P
S
S
S
S
L
L
I
I
V
V
H
H
1080
|
F
F
N
N
E
E
V
V
I
I
G
G
R
R
G
G
H
H
F
F
1090
|
G
G
C
C
V
V
Y
Y
H
H
G
G
T
T
L
L
L
L
D
D
1100
|
N
N
D
D
G
G
K
K
K
K
I
I
H
H
C
C
A
A
V
V
1110
|
K
K
S
S
L
L
N
N
R
R
I
I
T
T
D
D
I
I
G
G
1120
|
E
E
V
V
S
S
Q
Q
F
F
L
L
T
T
E
E
G
G
I
I
1130
|
I
I
M
M
K
K
D
D
F
F
S
S
H
H
P
P
N
N
V
V
1140
|
L
L
S
S
L
L
L
L
G
G
I
I
C
C
L
L
R
R
S
S
1150
|
E
E
G
G
S
S
P
P
L
L
V
V
V
V
L
L
P
P
Y
Y
1160
|
M
M
K
K
H
H
G
G
D
D
L
L
R
R
N
N
F
F
I
I
1170
|
R
R
N
N
E
E
T
T
H
H
N
N
P
P
T
T
V
V
K
K
1180
|
D
D
L
L
I
I
G
G
F
F
G
G
L
L
Q
Q
V
V
A
A
1190
|
K
K
G
G
M
M
K
K
F
Y
L
L
A
A
S
S
K
K
K
K
1200
|
F
F
V
V
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
1210
|
C
C
M
M
L
L
D
D
E
E
K
K
F
F
T
T
V
V
K
K
1220
|
V
V
A
A
D
D
F
F
G
G
L
L
A
A
R
R
D
V
M
M
1230
|
Y
Y
D
D
K
K
E
E
F
Y
D
Y
S
S
V
V
H
H
N
N
1240
|
K
K
T
T
G
G
A
A
K
K
L
L
P
P
V
V
K
K
W
W
1250
|
M
M
A
A
L
L
E
E
S
S
L
L
Q
Q
T
T
Q
Q
K
K
1260
|
F
F
T
T
T
T
K
K
S
S
D
D
V
V
W
W
S
S
F
F
1270
|
G
G
V
V
L
L
L
L
W
W
E
E
L
L
M
M
T
T
R
R
1280
|
G
G
A
A
P
P
P
P
Y
Y
P
P
D
D
V
V
N
N
T
T
1290
|
F
F
D
D
I
I
T
T
V
V
Y
Y
L
L
L
L
Q
Q
G
G
1300
|
R
R
R
R
L
L
L
L
Q
Q
P
P
E
E
Y
Y
C
C
P
P
1310
|
D
D
P
P
L
L
Y
Y
E
E
V
V
M
M
L
L
K
K
C
C
1320
|
W
W
H
H
P
P
K
K
A
A
E
E
M
M
R
R
P
P
S
S
1330
|
F
F
S
S
E
E
L
L
V
V
S
S
R
R
I
I
S
S
A
A
1340
|
I
I
F
F
S
S
T
T
F
F
I
I
G
G
E
-
H
-
Y
-
1350
|
V
-
H
-
V
-
N
-
A
-
T
-
Y
-
V
-
N
-
V
-
1360
|
K
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | |||||||||
| 293T cells | Breast | Homo sapiens (Human) | CVCL_0063 | ||||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| Experiment for Molecule Alteration |
Western blot analysis | ||||||||||||
| Experiment for Drug Resistance |
MTS assay | ||||||||||||
| Mechanism Description | There is a patient with metastatic NSCLC with MET-mediated resistance to EGFR TKI who responded to treatment with a type I MET inhibitor, savolitinib, given in combination with a third-generation EGFR inhibitor, osimertinib. The patient then developed acquired resistance mediated by a novel MET kinase domain mutation. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
