Drug Information
Drug (ID: DG01552) and It's Reported Resistant Information
| Name |
Tepotinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tepotinib; 1100598-32-0; EMD-1214063; EMD 1214063; MSC2156119J; UNII-1IJV77EI07; EMD1214063; MSC2156119; 1IJV77EI07; 3-[1-[[3-[5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl]phenyl]methyl]-6-oxopyridazin-3-yl]benzonitrile; Tepotinib (EMD 1214063); MSC-2156119J; MSC-2156119; 3-(1-(3-(5-((1-methylpiperidin-4-yl)methoxy)pyrimidin-2-yl)benzyl)-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; 3-[1-(3-{5-[(1-Methylpiperidin-4-Yl)methoxy]pyrimidin-2-Yl}benzyl)-6-Oxo-1,6-Dihydropyridazin-3-Yl]benzonitrile; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo-3-pyridazinyl)-; Tepmetko; Tepotinib [USAN]; Tepotinib (USAN/INN); MLS006010785; GTPL8293; SCHEMBL1296895; Tepotinib(EMD-1214063); CHEMBL3402762; AOB5729; DTXSID70149132; EX-A394; WHO 9934; BDBM50065457; MFCD18452823; NSC758244; NSC781256; NSC800945; s7067; ZINC43202335; CCG-269632; CS-0647; DB15133; NSC-758244; NSC-781256; NSC-800945; SB16609; compound 22 [PMID: 25736998]; NCGC00345793-01; NCGC00345793-08; AS-16915; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; HY-14721; QC-11720; SMR004701471; Y0358; D11717; J-002366; Q27088961; 1100598-30-8; 3-(1-(3-(5-((1-Methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)benzyl)-6-oxo-1,6-dihydro-3-pyridazinyl)benzonitrile; 3-(1-{3-[5-(1-methylpiperidin-4-yl-methoxy)pyrimidin-2-yl]benzyl}-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile; 3-(1-{3-[5-(1-methylpiperidin-4-ylmethoxy)pyrimidin-2-yl]benzyl}-6-oxo-1,6-dihydropyridazin-3-yl)benzonitrile; 3-[1,6-Dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]benzonitrile; 3E8
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
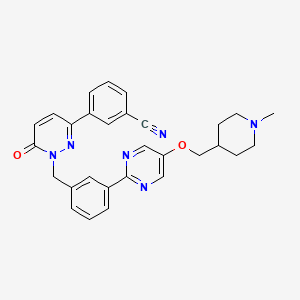
|
||||
| Target | PI3-kinase gamma (PIK3CG) | PK3CG_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
7
|
||||
| IsoSMILES |
CN1CCC(CC1)COC2=CN=C(N=C2)C3=CC=CC(=C3)CN4C(=O)C=CC(=N4)C5=CC=CC(=C5)C#N
|
||||
| InChI |
InChI=1S/C29H28N6O2/c1-34-12-10-21(11-13-34)20-37-26-17-31-29(32-18-26)25-7-3-5-23(15-25)19-35-28(36)9-8-27(33-35)24-6-2-4-22(14-24)16-30/h2-9,14-15,17-18,21H,10-13,19-20H2,1H3
|
||||
| InChIKey |
AHYMHWXQRWRBKT-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.Y1003F (c.3008A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | |
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | |
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | |
| Experiment for Molecule Alteration |
Direct sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D1010Y (c.3028G>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | |
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | |
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | |
| Experiment for Molecule Alteration |
Direct sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
