Drug Information
Drug (ID: DG01548) and It's Reported Resistant Information
| Name |
Momelotinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cyt387; 1056634-68-4; MOMELOTINIB; Cyt-387; CYT 387; N-(Cyanomethyl)-4-(2-((4-morpholinophenyl)amino)pyrimidin-4-yl)benzamide; CYT 11387; UNII-6O01GMS00P; N-(cyanomethyl)-4-(2-(4-morpholinophenylamino)pyrimidin-4-yl)benzamide; Momelotinib (CYT387); N-(cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]benzamide; 6O01GMS00P; CHEMBL1078178; CYT11387; N-(cyanomethyl)-4-(2-((4-(4-morpholinyl)phenyl)amino)-4-pyrimidinyl)benzamide; N-(Cyanomethyl)-4-(2-((4-morpholinophenyl)-amino)pyrimidin-4-yl)benzamide; CYT-11387; MFCD16038899; GS-0387; LM-1149; N-(Cyanomethyl)-4-(2-((4-(morpholin-4-yl)phenyl)amino)pyrimidin-4-yl)benzamide; N-(cyanomethyl)-4-(2-{[4-(morpholin-4-yl)phenyl]amino}pyrimidin-4-yl)benzamide; Benzamide, N-(cyanomethyl)-4-(2-((4-(4-morpholinyl)phenyl)amino)-4-pyrimidinyl)-; Momelotinib [USAN:INN]; momelotinibum; benzamide, n-(cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]-; C87; Momelotinib (USAN/INN); MLS006011154; GTPL7791; SCHEMBL2237234; CHEBI:91407; AOB4616; EX-A260; QCR-259; SYN1035; HMS3244K19; HMS3244K20; HMS3244L19; HMS3295I01; HMS3655D12; HMS3744C15; BCP02328; CYT-0387; CYT387, CYT11387; CYT387,CYT 11387; Momelotinib (CYT387,CYT-387); BDBM50311017; NSC767598; NSC800800; s2219; ZINC43199890; AKOS015904624; N-(Cyanomethyl)-4-[2-[(4-morpholinophenyl)amino]-4-pyrimidinyl]benzamide; BCP9000570; CCG-264946; CS-0053; DB11763; LS41099; NSC-767598; NSC-800800; SB14602; NCGC00244257-01; NCGC00244257-08; AC-23162; AS-19389; HY-10961; SMR004702928; SY060668; FT-0755949; SW219679-1; X7506; D10315; A857769; Q252602; J-001468; J-523159; BRD-K87737963-001-01-1; N-(cyanomethyl)-4-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl]benzamide; N-(cyanomethyl)-4-[2-[4-(4-morpholinyl)anilino]-4-pyrimidinyl]benzamide; N-(cyanomethyl)-4-{2-[4-(morpholin-4-yl)anilino]pyrimidin-4-yl}benzamide; Benzamide,N-(cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]-; N-(Cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]benzamide;CYT387
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
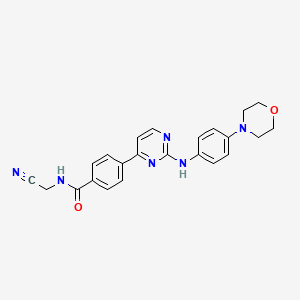
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | PI3-kinase gamma (PIK3CG) | PK3CG_HUMAN | [1] | ||
| Serine/threonine-protein kinase mTOR (mTOR) | MTOR_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
C1COCCN1C2=CC=C(C=C2)NC3=NC=CC(=N3)C4=CC=C(C=C4)C(=O)NCC#N
|
||||
| InChI |
InChI=1S/C23H22N6O2/c24-10-12-25-22(30)18-3-1-17(2-4-18)21-9-11-26-23(28-21)27-19-5-7-20(8-6-19)29-13-15-31-16-14-29/h1-9,11H,12-16H2,(H,25,30)(H,26,27,28)
|
||||
| InChIKey |
ZVHNDZWQTBEVRY-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Resistant Disease | Hematologic Cancer [ICD-11: MG24.Y] | |||
| Molecule Alteration | Missense mutation | p.R867Q (c.2600G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
WST-1 cell proliferation assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
