Drug Information
Drug (ID: DG01545) and It's Reported Resistant Information
| Name |
MK-2206
|
||||
|---|---|---|---|---|---|
| Synonyms |
MK-2206; 1032349-93-1; UNII-51HZG6MP1K; MK 2206; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one; MK2206; 51HZG6MP1K; CHEMBL1079175; CHEBI:67271; NCGC00186465-01; DSSTox_CID_28874; DSSTox_RID_83143; DSSTox_GSID_48948; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; 1,2,4-Triazolo[3,4-f][1,6]naphthyridin-3(2H)-one, 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-;1,2,4-Triazolo[3,4-f][1,6]naphthyridin-3(2H)-one, 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-;8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-ol; 8-(4-(1-Aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthy ridin-3(2H)-one; 8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; CAS-1032350-13-2; 1032349-77-1; MK-2206 free base; SCHEMBL530721; GTPL7945; DTXSID3048948; BCP20200; Tox21_113368; BDBM50313650; NSC756656; NSC800794; ZINC36382821; AKOS032945686; Tox21_113368_1; BCP9000938; MK 2206;MK2206; NSC-756656; NSC-800794; SB16805; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-1,2,4-Triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; NCGC00186465-02; NCGC00186465-03; NCGC00186465-04; NCGC00186465-08; DB-058959; FT-0738942; A25240; MK2206;MK 2206;MK-2206 dihydrochloride; cas:1032350-13-2;MK 2206; BRD-K68065987-300-02-6; Q25100065; 1,2,4-Triazolo(3,4-f)(1,6)naphthyridin-3(2H)-one, 8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-; 8-[4-(1-Aminocyclobutyl)phenyl]-9-phenyl-1,2,4-triazolo[3,4-f][1,6]naphthyridin-3(2H)-one dihydrochloride;MK-2206 Dihydrochloride; 8-[4-(1-Aminocyclobutyl)phenyl]-9-phenyl[1,2,4]triazolo[3,4-f]-1,6-naphthyridin-3(2H)-one
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
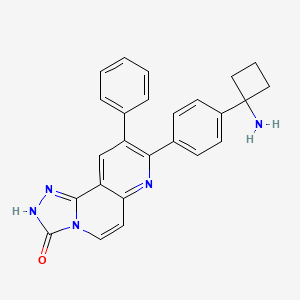
|
||||
| Target | MAPK/ERK kinase kinase (MAP3K) | NOUNIPROTAC | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
3
|
||||
| IsoSMILES |
C1CC(C1)(C2=CC=C(C=C2)C3=C(C=C4C(=N3)C=CN5C4=NNC5=O)C6=CC=CC=C6)N
|
||||
| InChI |
InChI=1S/C25H21N5O/c26-25(12-4-13-25)18-9-7-17(8-10-18)22-19(16-5-2-1-3-6-16)15-20-21(27-22)11-14-30-23(20)28-29-24(30)31/h1-3,5-11,14-15H,4,12-13,26H2,(H,29,31)
|
||||
| InChIKey |
ULDXWLCXEDXJGE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: GTPase KRas (KRAS) | [2] | ||||||||||||
| Sensitive Disease | Pancreatic ductal adenocarcinoma [ICD-11: 2C10.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.G12D (c.35G>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.40 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.10 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
0
|
G
-
M
M
T
T
E
E
Y
Y
K
K
L
L
V
V
V
V
V
V
10
|
G
G
A
A
G
D
G
G
V
V
G
G
K
K
S
S
A
A
L
L
20
|
T
T
I
I
Q
Q
L
L
I
I
Q
Q
N
N
H
H
F
F
V
V
30
|
D
D
E
E
Y
Y
D
D
P
P
T
T
I
I
E
E
D
D
S
S
40
|
Y
Y
R
R
K
K
Q
Q
V
V
V
V
I
I
D
D
G
G
E
E
50
|
T
T
C
C
L
L
L
L
D
D
I
I
L
L
D
D
T
T
A
A
60
|
G
G
Q
Q
E
E
E
E
Y
Y
S
S
A
A
M
M
R
R
D
D
70
|
Q
Q
Y
Y
M
M
R
R
T
T
G
G
E
E
G
G
F
F
L
L
80
|
C
C
V
V
F
F
A
A
I
I
N
N
N
N
T
T
K
K
S
S
90
|
F
F
E
E
D
D
I
I
H
H
H
H
Y
Y
R
R
E
E
Q
Q
100
|
I
I
K
K
R
R
V
V
K
K
D
D
S
S
E
E
D
D
V
V
110
|
P
P
M
M
V
V
L
L
V
V
G
G
N
N
K
K
C
C
D
D
120
|
L
L
P
P
S
S
R
R
T
T
V
V
D
D
T
T
K
K
Q
Q
130
|
A
A
Q
Q
D
D
L
L
A
A
R
R
S
S
Y
Y
G
G
I
I
140
|
P
P
F
F
I
I
E
E
T
T
S
S
A
A
K
K
T
T
R
R
150
|
Q
Q
G
R
V
V
D
E
D
D
A
A
F
F
Y
Y
T
T
L
L
160
|
V
V
R
R
E
E
I
I
R
R
K
Q
H
Y
K
R
E
L
K
K
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Mechanism Description | The missense mutation p.G12D (c.35G>A) in gene KRAS cause the sensitivity of MK-2206 by unusual activation of pro-survival pathway | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: PI3-kinase alpha (PIK3CA) | [1] | |||
| Sensitive Disease | Breast adenocarcinoma [ICD-11: 2C60.1] | |||
| Molecule Alteration | Missense mutation | p.E545K (c.1633G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| Experiment for Molecule Alteration |
Immunoblotting analysis | |||
| Experiment for Drug Resistance |
Soft-agar colony formation assay | |||
| Mechanism Description | The missense mutation p.E545K (c.1633G>A) in gene PIK3CA cause the sensitivity of MK-2206 by aberration of the drug's therapeutic target | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
