Drug Information
Drug (ID: DG01525) and It's Reported Resistant Information
| Name |
IRAK-1 or IRAK-4 inhibitors
|
||||
|---|---|---|---|---|---|
| Synonyms |
IRAK-1-4 Inhibitor I; 509093-47-4; IRAK-1/4 Inhibitor; IRAK-1/4 Inhibitor I; N-[1-(2-morpholin-4-ylethyl)benzimidazol-2-yl]-3-nitrobenzamide; Interleukin-1 Receptor-Associated-Kinase-1/4 Inhibitor; CHEMBL379787; N-(1-(2-morpholinoethyl)-1H-benzo[d]imidazol-2-yl)-3-nitrobenzamide; N-[1-[2-(4-morpholinyl)ethyl]-1H-benzimidazol-2-yl]-3-nitrobenzamide; IRAK-1-4 Inhibitor; IRAK1/4 Inhibitor I; MLS006010636; GTPL5990; SCHEMBL3600534; DTXSID50475143; HMS3229G05; HMS3263A20; HMS3744A07; BCP16164; EX-A1254; Tox21_501049; 3747AH; BDBM50184706; MFCD09752602; s6598; ZINC16052821; AKOS026750497; Benzamide, N-[1-[2-(4-morpholinyl)ethyl]-1H-benzimidazol-2-yl]-3-nitro-; CCG-206760; CS-0704; LP01049; SDCCGSBI-0086683.P002; NCGC00186035-01; NCGC00186035-02; NCGC00186035-03; NCGC00186035-04; NCGC00186035-10; NCGC00261734-01; BS-17437; HY-13329; SMR001456469; J3.545.430F; F17398; IRAK-1/4 Inhibitor I, >=98% (HPLC), solid; Q27078097; 1-[2-(4-Morpholinyl)ethyl]-2-(3-nitrobenzoylamino)-1H-benzoimidazole; 3-nitro-N-(1-(2-morpholin-4-yl-ethyl)-1H-benzoimidazol-2-yl)-benzamide; N-{1-[2-(morpholin-4-yl)ethyl]-1H-1,3-benzodiazol-2-yl}-3-nitrobenzamide
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
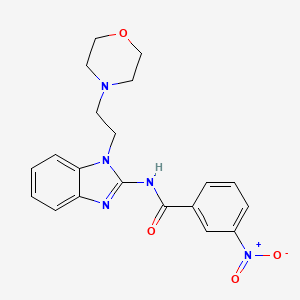
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
5
|
||||
| IsoSMILES |
C1COCCN1CCN2C3=CC=CC=C3N=C2NC(=O)C4=CC(=CC=C4)[N+](=O)[O-]
|
||||
| InChI |
InChI=1S/C20H21N5O4/c26-19(15-4-3-5-16(14-15)25(27)28)22-20-21-17-6-1-2-7-18(17)24(20)9-8-23-10-12-29-13-11-23/h1-7,14H,8-13H2,(H,21,22,26)
|
||||
| InChIKey |
QTCFYQHZJIIHBS-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Myeloid differentiation primary response protein MyD88 (MYD88) | [1] | |||
| Sensitive Disease | Mantle cell lymphoma [ICD-11: 2A85.0] | |||
| Molecule Alteration | Missense mutation | p.L265P (c.794T>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Blood | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Mechanism Description | The missense mutation p.L265P (c.794T>C) in gene MYD88 cause the sensitivity of IRAK-1 or IRAK-4 inhibitors by unusual activation of pro-survival pathway | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
