Drug Information
Drug (ID: DG01520) and It's Reported Resistant Information
| Name |
RAF-265
|
||||
|---|---|---|---|---|---|
| Synonyms |
RAF265; 927880-90-8; CHIR-265; RAF 265; RAF-265; RAF265(CHIR-265); CHIR 265; RAF265 (CHIR-265); 1-Methyl-5-[[2-[5-(trifluoromethyl)-1h-imidazol-2-yl]-4-pyridinyl]oxy]-n-[4-(trifluoromethyl)phenyl]-1h-benzimidazol-2-amine; 1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]pyridin-4-yl]oxy-N-[4-(trifluoromethyl)phenyl]benzimidazol-2-amine; CHIR265; UNII-8O434L3768; RAF-265 (CHIR-265); 8O434L3768; 1-Methyl-5-({2-[5-(Trifluoromethyl)-1h-Imidazol-2-Yl]pyridin-4-Yl}oxy)-N-[4-(Trifluoromethyl)phenyl]-1h-Benzimidazol-2-Amine; 1-methyl-5-(2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-2-amine; 1-Methyl-5-[[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]-4-pyridinyl]-oxy]-N-[4-(trifluoromethyl)phenyl]-1H-benzimidazol-2-amine; NVP-RAF265; SCHEMBL686452; CHEMBL558752; GTPL5674; BDBM31088; C24H16F6N6O; CHEBI:91451; EX-A072; cid_11656518; HMS3655B06; HMS3672I21; AMY24172; AOB87162; RAF265 - CHIR-265; MFCD16659061; NSC754357; NSC800861; s2161; ZINC18710085; CHIR 265 (RAF 265); AKOS025117575; CCG-264703; CS-0232; DB05984; EX-8671; NSC-754357; NSC-800861; SB16578; NCGC00250407-01; NCGC00250407-04; 1-methyl-5-((2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yl)oxy)-N-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-2-amine; AC-30291; AS-56738; HY-10248; QC-11760; FT-0746327; SW219923-1; X7407; A25488; J-504965; Q27075961; [1-methyl-5-[[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]-4-pyridyl]oxy]benzimidazol-2-yl]-[4-(trifluoromethyl)phenyl]amine; {1-Methyl-5-[2-(5-trifluoromethyl-1H-imidazol-2-yl)-pyridin-4-yloxy]-1H-benzo-imidazol-2-yl}-(4-trifluoromethyl-phenyl)-amine; {1-Methyl-5-[2-(5-trifluoromethyl-1H-imidazol-2-yl)-pyridin-4-yloxy]-1H-benzoimidazol-2-yl}-(4-trifluoromethyl-phenyl)-amine; 1-methyl-5-({2-[4-(trifluoromethyl)-1H-imidazol-2-yl]pyridin-4-yl}oxy)-N-[4-(trifluoromethyl)phenyl]-1H-1,3-benzodiazol-2-amine; 1-methyl-5-[[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]-4-pyridinyl]oxy]-N-[4-(trifluoromethyl)phenyl]-2-benzimidazolamine; 1-methyl-5-[2-(5-trifluoromethyl-1H-imidazol-2-yl)pyridin-4-yloxy]-N-(4-trifluoromethylphenyl)-1H-benzo[d]imidazol-2-amine; 1H-Benzimidazol-2-amine, 1-methyl-5-((2-(5-(trifluoromethyl)-1H-imidazol-2-yl)-4-pyridinyl)oxy)-N-(4-(trifluoromethyl)phenyl)-; 1H-Benzimidazol-2-amine, 1-methyl-5-[[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]-4-pyridinyl]oxy]-N-[4-(trifluoromethyl)phenyl]; 55J; CHIR-265;1-methyl-5-(2-(5-(trifluoromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-2-amine
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
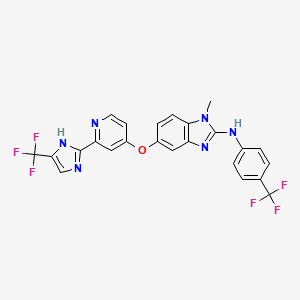
|
||||
| Target | ALK tyrosine kinase receptor (ALK) | ALK_HUMAN | [2] | ||
| HGF/Met signaling pathway (HGF/Met pathway) | NOUNIPROTAC | [2] | |||
| Proto-oncogene c-Met (MET) | MET_HUMAN | [2] | |||
| Proto-oncogene c-Ros (ROS1) | ROS1_HUMAN | [2] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
5
|
||||
| IsoSMILES |
CN1C2=C(C=C(C=C2)OC3=CC(=NC=C3)C4=NC=C(N4)C(F)(F)F)N=C1NC5=CC=C(C=C5)C(F)(F)F
|
||||
| InChI |
InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34)
|
||||
| InChIKey |
YABJJWZLRMPFSI-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [2] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Revealed Based on the Cell Line Data | ||||||||||||
| In Vitro Model | HEK293 cells | Kidney | Homo sapiens (Human) | CVCL_0045 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| In Vivo Model | Nude mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
BRAF kinase assay | ||||||||||||
| Experiment for Drug Resistance |
Flow cytometry assay | ||||||||||||
| Mechanism Description | The missense mutation p.V600E (c.1799T>A) in gene BRAF cause the sensitivity of RAF-265 by aberration of the drug's therapeutic target | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Serine/threonine-protein kinase B-raf (BRAF) | [1] | ||||||||||||
| Sensitive Disease | Melanoma [ICD-11: 2C30.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V600E (c.1799T>A) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.55 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 3.20 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
420
|
M
M
D
D
R
R
G
G
S
S
H
H
H
H
H
H
H
H
H
H
430
|
H
H
G
G
S
S
E
E
D
D
R
R
N
N
R
R
M
M
K
K
440
|
T
T
L
L
G
G
R
R
R
R
D
D
S
S
S
S
D
D
D
D
450
|
W
W
E
E
I
I
P
P
D
D
G
G
Q
Q
I
I
T
T
V
V
460
|
G
G
Q
Q
R
R
I
I
G
G
S
S
G
G
S
S
F
F
G
G
470
|
T
T
V
V
Y
Y
K
K
G
G
K
K
W
W
H
H
G
G
D
D
480
|
V
V
A
A
V
V
K
K
M
M
L
L
N
N
V
V
T
T
A
A
490
|
P
P
T
T
P
P
Q
Q
Q
Q
L
L
Q
Q
A
A
F
F
K
K
500
|
N
N
E
E
V
V
G
G
V
V
L
L
R
R
K
K
T
T
R
R
510
|
H
H
V
V
N
N
I
I
L
L
L
L
F
F
M
M
G
G
Y
Y
520
|
S
S
T
T
K
K
P
P
Q
Q
L
L
A
A
I
I
V
V
T
T
530
|
Q
Q
W
W
C
C
E
E
G
G
S
S
S
S
L
L
Y
Y
H
H
540
|
H
H
L
L
H
H
I
I
I
I
E
E
T
T
K
K
F
F
E
E
550
|
M
M
I
I
K
K
L
L
I
I
D
D
I
I
A
A
R
R
Q
Q
560
|
T
T
A
A
Q
Q
G
G
M
M
D
D
Y
Y
L
L
H
H
A
A
570
|
K
K
S
S
I
I
I
I
H
H
R
R
D
D
L
L
K
K
S
S
580
|
N
N
N
N
I
I
F
F
L
L
H
H
E
E
D
D
L
L
T
T
590
|
V
V
K
K
I
I
G
G
D
D
F
F
G
G
L
L
A
A
T
T
600
|
V
E
K
K
S
S
R
R
W
W
S
S
G
G
S
S
H
H
Q
Q
610
|
F
F
E
E
Q
Q
L
L
S
S
G
G
S
S
I
I
L
L
W
W
620
|
M
M
A
A
P
P
E
E
V
V
I
I
R
R
M
M
Q
Q
D
D
630
|
K
K
N
N
P
P
Y
Y
S
S
F
F
Q
Q
S
S
D
D
V
V
640
|
Y
Y
A
A
F
F
G
G
I
I
V
V
L
L
Y
Y
E
E
L
L
650
|
M
M
T
T
G
G
Q
Q
L
L
P
P
Y
Y
S
S
N
N
I
I
660
|
N
N
N
N
R
R
D
D
Q
Q
I
I
I
I
F
F
M
M
V
V
670
|
G
G
R
R
G
G
Y
Y
L
L
S
S
P
P
D
D
L
L
S
S
680
|
K
K
V
V
R
R
S
S
N
N
C
C
P
P
K
K
A
A
M
M
690
|
K
K
R
R
L
L
M
M
A
A
E
E
C
C
L
L
K
K
K
K
700
|
K
K
R
R
D
D
E
E
R
R
P
P
L
L
F
F
P
P
Q
Q
710
|
I
I
L
L
A
A
S
S
I
I
E
E
L
L
L
L
A
A
R
R
720
|
S
S
L
L
P
P
K
K
I
I
H
H
R
R
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Skin sample | N.A. | |||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
