Drug Information
Drug (ID: DG01517) and It's Reported Resistant Information
| Name |
Tivantinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tivantinib; 905854-02-6; ARQ 197; ARQ-197; ARQ197; (3R,4R)-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; UNII-PJ4H73IL17; Tivantinib (ARQ 197); PJ4H73IL17; 1000873-98-2; (3R,4R)-3-(5,6-Dihydro-4H-pyrrolo(3,2,1-ij)quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; 905853-99-8; rel-(3R,4R)-3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-2,5-pyrrolidinedione; Tivantinib [USAN:INN]; Tivantinib; ARQ197; ARQ 197 (Tivantinib); Tivantinib (ARQ-197); SCHEMBL44944; Tivantinib (JAN/USAN/INN); GTPL7948; CHEMBL2103882; CHEBI:91398; DTXSID60920316; QCR-102; ARQ-197,Tivantinib, ARQ197; ARQ197; ARQ-197;Tivantinib; BCPP000042; AOB87130; BCP02529; EX-A2169; CAS:905854-02-6;Tivantinib; BDBM50146168; NSC758242; NSC800951; s2753; AKOS022182739; ZINC100016063; CCG-268285; DB12200; NSC-758242; NSC-800951; (-)-trans-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; AC-25009; AS-16991; HY-50686; J3.496.311H; C77073; D10173; E82820; A857990; J-501255; BRD-K33379087-001-01-8; Q17123902; (+/-)-trans-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4(1H-indol-3-yl)pyrrolidine-2,5-dione; (+/-)-trans-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl) pyrrolidine-2,5-dione; (+/-)-trans-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; (_)-trans-3-(5,6-dihydro-4h-pyrrolo[3,2,1-ij]quinolin-1-yl)-4(1h-indol-3-yl)pyrrolidine-2,5-dione; (3R,4R)-3-(1-azatricyclo[6.3.1.04,12]dodeca-2,4,6,8(12)-tetraen-3-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; (3R,4R)-3-(2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinolin-6-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; (3R,4R)-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione; (3R,4R)-3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-yl)-4-(1H-indole-3-yl)pyrrolidine-2,5-dione; 2,5-Pyrrolidinedione, 3-(5,6-dihydro-4H-pyrrolo(3,2,1-ij)quinolin-1-yl)-4-(1H-indol-3-yl)-, (3R,4R)-; 2,5-Pyrrolidinedione,3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-,(3R,4R)-rel-; 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-5-hydroxy-4-(1H-indol-3-yl)-3,4-dihydro-2H-pyrrol-2-one; TIV; trans 3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
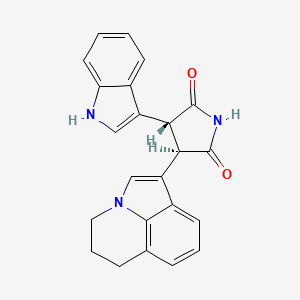
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Erbb2 tyrosine kinase receptor (HER2) | ERBB2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
2
|
||||
| IsoSMILES |
C1CC2=C3C(=CC=C2)C(=CN3C1)[C@H]4[C@@H](C(=O)NC4=O)C5=CNC6=CC=CC=C65
|
||||
| InChI |
InChI=1S/C23H19N3O2/c27-22-19(16-11-24-18-9-2-1-7-14(16)18)20(23(28)25-22)17-12-26-10-4-6-13-5-3-8-15(17)21(13)26/h1-3,5,7-9,11-12,19-20,24H,4,6,10H2,(H,25,27,28)/t19-,20-/m0/s1
|
||||
| InChIKey |
UCEQXRCJXIVODC-PMACEKPBSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D1010Y (c.3028G>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | |
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | |
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | |
| Experiment for Molecule Alteration |
Direct sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.Y1003F (c.3008A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 |
| WEHI-3 cells | Peripheral blood | Mus musculus (Mouse) | CVCL_3622 | |
| Hs746T cells | Skeletal muscle | Homo sapiens (Human) | CVCL_0333 | |
| Gp2-293 cells | Fetal kidney | Homo sapiens (Human) | CVCL_WI48 | |
| Experiment for Molecule Alteration |
Direct sequencing assay | |||
| Experiment for Drug Resistance |
CCK-8 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
