Drug Information
Drug (ID: DG01498) and It's Reported Resistant Information
| Name |
SU11274
|
||||
|---|---|---|---|---|---|
| Synonyms |
SU11274; 658084-23-2; Met Kinase Inhibitor; SU-11274; SU 11274; PKI-SU11274; SU-MI-2; CHEMBL261641; (3Z)-N-(3-Chlorophenyl)-3-({3,5-dimethyl-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol-2-yl}methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide; MFCD08276928; (Z)-N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide; (3Z)-N-(3-Chlorophenyl)-3-((3,5-dimethyl-4-((4-methylpiperazin-1-yl)carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide; (3Z)-N-(3-Chlorophenyl)-3-[[3,5-dimethyl-4-[(4-methyl-1-piperazinyl)carbonyl]-1H-pyrrol-2-yl]methylene]-2,3-dihydro-N-methyl-2-oxo-1H-indole-5-sulfonamide; (3z)-N-(3-Chlorophenyl)-3-({3,5-Dimethyl-4-[(4-Methylpiperazin-1-Yl)carbonyl]-1h-Pyrrol-2-Yl}methylene)-N-Methyl-2-Oxoindoline-5-Sulfonamide; (3Z)-N-(3-chlorophenyl)-3-({3,5-dimethyl-4-[(4-methylpiperazin-1-yl)carbonyl]-1H-pyrrol-2-yl}methylidene)-N-methyl-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide; (Z)-N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(1-methylpiperazine-4-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide; (3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylidene]-N-methyl-2-oxo-1H-indole-5-sulfonamide; C28H30ClN5O4S; SCHEMBL93711; SCHEMBL93713; MLS006010961; GTPL5057; AOB6383; DTXSID20429552; EX-A183; CHEBI:190974; BCPP000061; HMS3229G21; K00593a; BDBM50341636; NSC747693; s1080; ZINC16052811; AKOS015994564; CCG-206768; ES-0032; EX-5962; NSC-747693; QC-2155; NCGC00165902-01; NCGC00165902-04; AC-28396; SMR004702765; SU-011274; SU11274 (PKI-SU11274); SU 11274, >=98% (HPLC), powder; X7471; EC-000.2357; A15738; Met Kinase Inhibitor - CAS 658084-23-2; J-522999; BRD-K02965346-001-01-8; BRD-K02965346-001-07-5; Q27088888; (3Z)-N-(3-chlorophenyl)-3-[[3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl]methylidene]-N-methyl-2-oxo-1H-indole-5-sulonamide; 1h-indole-5-sulfonamide, n-(3-chlorophenyl)-3-[[3,5-dimethyl-4-[(4-methyl-1-piperazinyl)carbonyl]-1h-pyrrol-2-yl]methylene]-2,3-dihydro-n-methyl-2-oxo-, (3z)-; N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(1-methylpiperazine-4-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide; N-(3-chlorophenyl)-3-((3,5-dimethyl-4-(4-methylpiperazine-1-carbonyl)-1H-pyrrol-2-yl)methylene)-N-methyl-2-oxoindoline-5-sulfonamide; N-(3-Chlorophenyl)-n-methyl-3-[[3,5-dmethyl-4-[(4-methylpperazn-1-yl)carbonyl]-1h-pyrrol-2-yl]methylene]-2-oxo-2,3-dhydro-1h-ndole-5 -sulfonamde
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
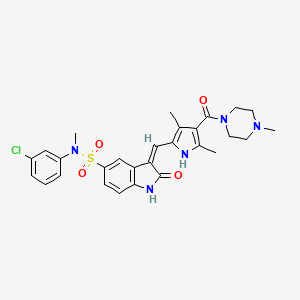
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[2]
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Proto-oncogene c-Met (MET) | MET_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
5
|
||||
| IsoSMILES |
CC1=C(NC(=C1C(=O)N2CCN(CC2)C)C)/C=C\\3/C4=C(C=CC(=C4)S(=O)(=O)N(C)C5=CC(=CC=C5)Cl)NC3=O
|
||||
| InChI |
InChI=1S/C28H30ClN5O4S/c1-17-25(30-18(2)26(17)28(36)34-12-10-32(3)11-13-34)16-23-22-15-21(8-9-24(22)31-27(23)35)39(37,38)33(4)20-7-5-6-19(29)14-20/h5-9,14-16,30H,10-13H2,1-4H3,(H,31,35)/b23-16-
|
||||
| InChIKey |
FPYJSJDOHRDAMT-KQWNVCNZSA-N
|
||||
| PubChem CID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [2] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.N375S (c.1124A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Mechanism Description | The missense mutation p.N375S (c.1124A>G) in gene MET cause the resistance of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.L1213V (c.3637C>G) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.L1213V (c.3637C>G) in gene MET cause the resistance of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.Y1248H (c.3742T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.Y1248H (c.3742T>C) in gene MET cause the resistance of SU11274 by aberration of the drug's therapeutic target | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.M1268T (c.3803T>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.M1268T (c.3803T>C) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V1110I (c.3328G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.V1110I (c.3328G>A) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.H1112L (c.3335A>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.H1112L (c.3335A>T) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V1206L (c.3616G>C) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.V1206L (c.3616G>C) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [3] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.V1238I (c.3712G>A) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.V1238I (c.3712G>A) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [2] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.E168D (c.504G>C) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Lung | N.A. | ||
| Mechanism Description | The missense mutation p.E168D (c.504G>C) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
| Key Molecule: Hepatocyte growth factor receptor (MET) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.H1112Y (c.3334C>T) |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | NIH3T3 cells | Embryo | Homo sapiens (Human) | CVCL_0594 |
| Experiment for Drug Resistance |
Flow cytometry assay | |||
| Mechanism Description | The missense mutation p.H1112Y (c.3334C>T) in gene MET cause the sensitivity of SU11274 by aberration of the drug's therapeutic target | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
