Drug Information
Drug (ID: DG01469) and It's Reported Resistant Information
| Name |
Tegafur
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tegafur; Ftorafur; 17902-23-7; Futraful; Fluorofur; Sinoflurol; Citofur; Fental; Neberk; Furofutran; Coparogin; Florafur; Fulfeel; Furafluor; Nitobanil; Exonal; Lamar; Lifril; Tefsiel C; 5-fluoro-1-(tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione; Franroze; Furflucil; Sunfral; Riol; FT-207; Phthorafur; 1-(2-Tetrahydrofuryl)-5-fluorouracil; 5-Fluoro-1-(tetrahydro-2-furyl)uracil; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-; MJF-12264; Fluorafur; Franrose; Sunfural; Fulaid; NSC-148958; 5-FLUORO-1-(TETRAHYDRO-2-FURFURYL)URACIL; FT 207; 5-Fluoro-1-(tetrahydro-2-furanyl)-2,4-pyrimidinedione; N1-(2-tetrahydrofuryl)-5-fluorouracil; 1-(Tetrahydrofuran-2-yl)-5-fluorouracil; 5-Fluoro-1-(tetrahydrofuran-2-yl)uracil; URACIL, 5-FLUORO-1-(TETRAHYDRO-2-FURYL)-; Uracil, 1-(tetrahydrofuran-2-yl)-5-fluoro-; MFCD00012351; 5-Fluoro-1-(tetrahydro-3-furyl)uracil; 1-(Tetrahydro-2-furanyl)-5-fluoro-2,4-pyrimidinedione; MLS000069497; CHEBI:32188; 1-(2-TETRAHYDROFORMYL)-5-FLUOROURACIL; 5-fluoro-1-(oxolan-2-yl)pyrimidine-2,4-dione; C8H9FN2O3; 5-fluoro-1-tetrahydrofuran-2-yl-pyrimidine-2,4-dione; NCGC00159418-02; NCGC00159418-05; SMR000059106; Racemic Ftorafur; DSSTox_CID_1305; DSSTox_RID_76070; DSSTox_GSID_21305; Phthorafur [Czech]; FT-207 (NSC 148958); Tegafurum; Tegafurum [INN-Latin]; NSC 148958; CAS-37076-68-9; CCRIS 2762; FT207; EINECS 241-846-2; BRN 0525766; Atillon; N1-(2'-Tetrahydrofuryl)-5-fluorouracil; 1-(Tetrahydro-2-furanyl)-5-fluorouracil; N(sub 1)-(2-Tetrahydrofuryl)-5-fluorouracil; Tegafur [USAN:INN:BAN:JAN]; N(sub 1)-(2'-Furanidyl)-5-fluouracil [Czech]; Atillon (TN); Tegafur ,(S); N(sub 1)-(2'-Furanidyl)-5-fluouracil; TS-1 (Salt/Mix); Opera_ID_1726; UPCMLD-DP063; SCHEMBL4552; 5-24-06-00285 (Beilstein Handbook Reference); MLS000759414; MLS001076521; MLS001424119; CHEMBL20883; Tegafur (JP17/USAN/INN); F-5-FU; UPCMLD-DP063:001; GTPL10513; DTXSID001009966; HMS1665I05; HMS2051B15; HMS2090K04; HMS2232E05; HMS3371H21; HMS3393B15; HMS3654P13; HMS3715D14; N1-(2'-Furanidyl)-5-fluouracil; 2,4(1H,3H)Pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-; 5-Fluoro-1-(tetrahydro-2-furanyl)-2,4(1H,3H)-pyrimidinedione; BCP22714; 5-fluoro-1-(tetrahydrofuran-2-yl); N1-(2'-Furanidyl)-5-fluorouracil; Tox21_111649; Tox21_301812; BBL027795; CCG-50110; STK528044; Tegafur, >=98% (HPLC), powder; 5-Fluoro-1-(2-tetrahydrofuryl)uracil; AKOS000121279; Tox21_111649_1; AC-2112; CCG-100959; CS-1128; DB09256; MCULE-1366455644; NC00209; 1-(tetrahydro-2-furyl)-5-fluorouracil; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-, didehydroderiv.; 5-fluoro-1-(2-tetrahydrofuranyl)uracil; N1 -(2-tetrahydrofuryl)-5-fluorouracil; NCGC00159418-04; NCGC00255222-01; 79107-97-4; 82294-77-7; AS-13528; HY-17400; I580; Tegafur (FT-207; NSC 148958); J356.722B; FT-0653732; FT-0654170; FT-0674829; FT-0693965; D01244; J10344; AB00572620-15; 902T237; A812417; Q413370; SR-01000639511; Q-201784; SR-01000639511-1; SR-01000639511-4; 5-fluoro-1-tetrahydro-furan-2-yl-1H-pyrimidine-2,4-dione; 5-Fluoro-1-tetrahydro-2-furanyl-2,4(1H,3H)-pyrimidinedione; 2, 4(1H,3H)-Pyrimidinedione, 5-fluoro-1-(tetrahydro-2-furanyl)-, (R)-
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
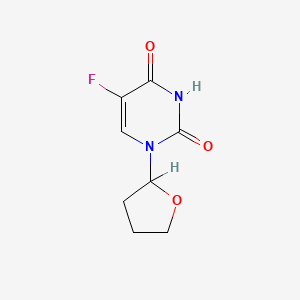
|
||||
| Target | Retinoic acid receptor gamma (RARG) | RARG_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
1
|
||||
| IsoSMILES |
C1CC(OC1)N2C=C(C(=O)NC2=O)F
|
||||
| InChI |
InChI=1S/C8H9FN2O3/c9-5-4-11(6-2-1-3-14-6)8(13)10-7(5)12/h4,6H,1-3H2,(H,10,12,13)
|
||||
| InChIKey |
WFWLQNSHRPWKFK-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Dihydropyrimidine dehydrogenase [NADP(+)] | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.I560S (c.1679T>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Key Molecule: Dihydropyrimidine dehydrogenase [NADP(+)] | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D949V (c.2846A>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
