Drug Information
Drug (ID: DG01465) and It's Reported Resistant Information
| Name |
GO6976
|
||||
|---|---|---|---|---|---|
| Synonyms |
Go 6976; 136194-77-9; GO6976; Go-6976; Goe 6976; UNII-B9IQO7JZ16; 5,6,7,13-Tetrahydro-13-Methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile; 3-(13-Methyl-5-oxo-6,7-dihydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12(13H)-yl)propanenitrile; B9IQO7JZ16; CHEMBL302449; C24H18N4O; CHEBI:51913; 12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole; 12H-Indolo(2,3-a)pyrrolo(3,4-c)carbazole-12-propanenitrile, 5,6,7,13-tetrahydro-13-methyl-5-oxo-; 12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile, 5,6,7,13-tetrahydro-13-methyl-5-oxo-; 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo[2,3-a]pyrolo[3,4-c]carbazole; 3-(13-methyl-5-oxo-5,6,7,13-tetrahydro-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12-yl)propanenitrile; Kinome_3630; Gouml 6976; CBiol_001871; BSPBio_001101; KBioGR_000441; KBioSS_000441; 12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole; 13-Methyl-5-oxo-5,6,7,13-tetrahydro-12H-indolo(2,3-a)pyrrolo(3,4-c)carbazole-12-propanenitrile; Goe-6976; BDBM3033; GTPL5973; QCR-45; SCHEMBL2175239; BCBcMAP01_000156; KBio2_000441; KBio2_003009; KBio2_005577; KBio3_000821; KBio3_000822; AOB4516; DTXSID70159731; Bio1_000157; Bio1_000646; Bio1_001135; Bio2_000381; Bio2_000861; HMS1362G03; HMS1792G03; HMS1990G03; HMS3229E13; HMS3403G03; BCP06797; ZINC1554668; 2437AH; 3-[methyl(oxo)[ ]yl]propanenitrile; EI-269; MFCD00236434; s7119; AKOS024457007; AT23580; CCG-206755; IDI1_002136; NCGC00163451-01; NCGC00163451-02; NCGC00163451-03; NCGC00163451-04; AS-16804; BG168444; HY-10183; CS-0002498; FT-0697606; EC-000.2399; G 6976, >=98% (HPLC), powder; A924795; G 6976; InSolution Go 6976 - CAS 136194-77-9; Go 6976 - CAS 136194-77-9; J-006821; BRD-K59304176-001-02-5; BRD-K59304176-001-03-3; Q27077832; 3-(13-methyl-5-oxo-5,6,7,13-tetrahydro-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-12-yl)propionitrile; 3-(23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10.0^{4,9.0^{11,15.0^{17,22]tricosa-1,4,6,8,10,15,17,19,21-nonaen-3-yl)propanenitrile; 3-(23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.02,10.04,9.011,15.017,22]tricosa-1,4,6,8,10,15,17,19,21-nonaen-3-yl)propanenitrile; 3-(9-methyl-1-oxo-2,3-dihydro-1H-indolo[2,3-a]pyrrolo[3,4-c]carbazol-8(9H)-yl)propanenitrile; 3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^{11,15}.0^{17,22}]tricosa-1(16),2(10),4(9),5,7,11(15),17,19,21-nonaen-3-yl}propanenitrile; 3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^{11,15}.0^{17,22}]tricosa-1(16),2(10),4,6,8,11(15),17,19,21-nonaen-3-yl}propanenitrile
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
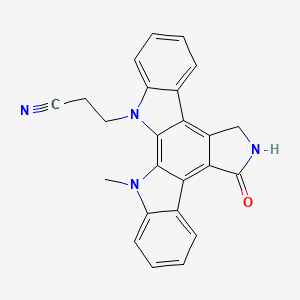
|
||||
| Target | Bacterial 30S ribosomal RNA (Bact 30S rRNA) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
2
|
||||
| IsoSMILES |
CN1C2=CC=CC=C2C3=C4C(=C5C6=CC=CC=C6N(C5=C31)CCC#N)CNC4=O
|
||||
| InChI |
InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29)
|
||||
| InChIKey |
VWVYILCFSYNJHF-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Sensitive Disease | Acute myeloid leukemia [ICD-11: 2A60.0] | |||
| Molecule Alteration | Missense mutation | p.V617F (c.1849G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TF-1 cells | Bone marrow | Homo sapiens (Human) | CVCL_0559 |
| HEL cells | Blood | Homo sapiens (Human) | CVCL_0001 | |
| Mo7E cells | Peripheral blood | Homo sapiens (Human) | CVCL_2106 | |
| FDCP1 cells | Bone marrow | Mus musculus (Mouse) | CVCL_2039 | |
| 32D cells | Bone marrow | Homo sapiens (Human) | CVCL_0118 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | The missense mutation p.V617F (c.1849G>T) in gene JAK2 cause the sensitivity of Go6976 by unusual activation of pro-survival pathway | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
