Drug Information
Drug (ID: DG01314) and It's Reported Resistant Information
| Name |
Anagrelide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Anagrelide; 68475-42-3; Anagrelida; Xagrid; Anagrelidum; 6,7-Dichloro-5,10-dihydroimidazo[2,1-b]quinazolin-2(3H)-one; UNII-K9X45X0051; CHEMBL760; 6,7-Dichloro-1,5-dihydroimidazo(2,1-b)quinazolin-2(3H)-one; CHEBI:142290; Imidazo[2,1-b]quinazolin-2(3H)-one, 6,7-dichloro-1,5-dihydro-; K9X45X0051; Anagrelide [INN:BAN]; Anagrelidum [INN-Latin]; Anagrelida [INN-Spanish]; 6,7-Dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)-one; 6,7-bis(chloranyl)-3,5-dihydro-1H-imidazo[2,1-b]quinazolin-2-one; Imidazo(2,1-b)quinazolin-2(3H)-one, 6,7-dichloro-1,5-dihydro-; C10H7Cl2N3O; HSDB 7325; BL 416201; Anagrelide (INN/BAN); BRN 0619582; 6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2[3H]-one; 6,7-Dichlor-1,5-dihydroimidazo(2,1-b)chinazolin-2(3H)-on; 6,7-dichloro-5,10-dihydro-3H-imidazo[2,1-b]quinazolin-2-one; SCHEMBL9411; BIDD:GT0711; GTPL7114; 6,7-dichloro-1,5-dihydroimidazo[2,1-]quinazolin-2(3H)-one; 6,7-dichloro-3,5-dihydroimidazo[2,1-b]quinazolin-2(1H)-one; HMS2089D21; HMS3715J06; HMS3742K13; BCP21314; HY-B0523; ZINC3871541; BDBM50000334; MFCD00866794; AKOS015899342; AKOS016340524; AC-3401; CCG-221242; DB00261; KS-5176; NCGC00161408-02; NCGC00161408-08; NCGC00247665-01; AS-14157; K139; SBI-0206823.P001; DB-055153; CS-0009495; FT-0602855; FT-0630776; D07455; AB00698496-05; AB00698496-07; AB00698496_08; AB01566808_01; 475A423; A915719; Q408163; 6,7-dichloro-3H,5H-imidazo[2,1-b]quinazolin-2-ol; BRD-K62200014-003-05-5; BRD-K62200014-003-08-9; 6,7-Dichloro-1,5-dihydro-imidazo[2,1-b]quinazolin-2-one; 6,7-dichloro-1h,5h-imidazo[2,1-b]quinazoline-2(3h)-one; 6,7-dichloro-1, 5-dihydroimidazo[2,1-b]quinazolin-2[3H]-one; 6,7-Dichloro-1,5-dihydroimidazo[2,1-b]-quinazolin-2(3H)-one; 6,7-dichloro-1,5dihydroimidazo[2,1-b]quinazolin-2[3H]-one; 6,7-dichloro-1H,2H,3H,5H-imidazolidino[2,1-b]quinazolin-2-one; Imidazo[2,1-b]quinazolin-2(3H)-one,6,7-dichloro-1,5-dihydro-; 6,7-di-chloro-1,5-dihydroimidazo[2,1-b]quinazolin-2[3 H ]-one base; 6,7-Dichloro-1,5-dihydro-imidazo[2,1-b]quinazolin-2-one(anagrelide); 6,7-Dichloro-1,5-dihydro-imidazo[2,1-b]quinazolin-2-one(BL-4162A); J33
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
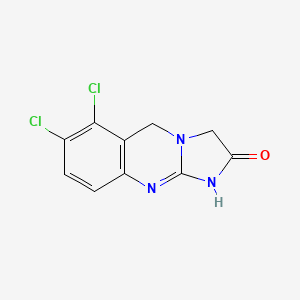
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(2 diseases)
[1]
[1]
|
||||
| Target | Phosphodiesterase 3A (PDE3A) | PDE3A_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C10H7Cl2N3O
|
||||
| IsoSMILES |
C1C2=C(C=CC(=C2Cl)Cl)N=C3N1CC(=O)N3
|
||||
| InChI |
1S/C10H7Cl2N3O/c11-6-1-2-7-5(9(6)12)3-15-4-8(16)14-10(15)13-7/h1-2H,3-4H2,(H,13,14,16)
|
||||
| InChIKey |
OTBXOEAOVRKTNQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: L1 cell adhesion molecule (L1CAM) | [1] | |||
| Resistant Disease | Ovarian cancer [ICD-11: 2C73.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Ovarian cancer [ICD-11: 2C73] | |||
| The Specified Disease | Ovarian cancer | |||
| The Studied Tissue | Blood | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 2.31E-10 Fold-change: -2.89E-01 Z-score: -6.73E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| In Vitro Model | 22RV1 cells | Prostate | Homo sapiens (Human) | CVCL_1045 |
| Experiment for Molecule Alteration |
Puromycin selection and monitored regularly for the maintenance of L1 silencing assay | |||
| Experiment for Drug Resistance |
Migration assay | |||
| Mechanism Description | With OVCAR3 cells treated with anagrelide, 2-hydroxy-5-fluoropyrimidine and mestranol , the gap width closure was seen from 48 h onward at all concentrations tested. Similar results were obtained with U251 cells, and L1's metastatic potential is further evidenced by its promotion of epithelial-mesenchymal transition, endothelial cell transcytosis and resistance to chemo- and radiotherapy. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: L1 cell adhesion molecule (L1CAM) | [1] | |||
| Resistant Disease | Glioblastoma [ICD-11: 2A00.02] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Brain cancer [ICD-11: 2A00] | |||
| The Specified Disease | Brain cancer | |||
| The Studied Tissue | Nervous tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 5.83E-128 Fold-change: -2.68E-01 Z-score: -2.75E+01 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell migration | Activation | hsa04670 | |
| In Vitro Model | MDCK cells | Kidney | Canis lupus familiaris (Dog) (Canis familiaris) | CVCL_0422 |
| Experiment for Molecule Alteration |
Puromycin selection and monitored regularly for the maintenance of L1 silencing assay | |||
| Experiment for Drug Resistance |
Migration assay | |||
| Mechanism Description | With OVCAR3 cells treated with anagrelide, 2-hydroxy-5-fluoropyrimidine and mestranol , the gap width closure was seen from 48 h onward at all concentrations tested. Similar results were obtained with U251 cells, and L1's metastatic potential is further evidenced by its promotion of epithelial-mesenchymal transition, endothelial cell transcytosis and resistance to chemo- and radiotherapy. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
