Drug Information
Drug (ID: DG01166) and It's Reported Resistant Information
| Name |
Axitinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Axitinib; 319460-85-0; AG-013736; Inlyta; AG 013736; (E)-N-Methyl-2-((3-(2-(pyridin-2-yl)vinyl)-1H-indazol-6-yl)thio)benzamide; UNII-C9LVQ0YUXG; AG-13736; AG013736; C9LVQ0YUXG; N-methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide; CHEBI:66910; (E)-N-methyl-2-(3-(2-(pyridin-2-yl)vinyl)-1H-indazol-6-ylthio)benzamide; N-methyl-2-[[3-[(E)-2-pyridin-2-ylethenyl]-1H-indazol-6-yl]sulfanyl]benzamide; MFCD09837898; NSC757441; N-methyl-2-({3-[(E)-2-(pyridin-2-yl)ethenyl]-1H-indazol-6-yl}sulfanyl)benzamide; NSC-757441; NCGC00241108-01; S1005; DSSTox_CID_28975; DSSTox_RID_83240; DSSTox_GSID_49049; Axitinib (AG 013736); C22H18N4OS; N-methyl-2-({3-[(E)-2-(pyridin-2-yl)vinyl]-1H-indazol-6-yl}sulfanyl)benzamide; CAS-319460-85-0; axitinibum; Axitinib [USAN:INN:JAN]; 4agc; Inlyta (TN); AG13736; 4ag8; Axitinib (JAN/USAN); AG-013736;Axitinib; Axitinib,AG-013736; MLS006010164; SCHEMBL172918; GTPL5659; Axitinib, >=98% (HPLC); CHEMBL1289926; DTXSID3049049; N-methyl-2-[[3-[2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]benzamide; SCHEMBL22930506; BDBM25117; CHEBI:94568; EX-A337; QCR-109; SYN1014; BCPP000372; AOB87786; BCP01371; ZINC3816287; Tox21_113597; NSC799341; AKOS015902898; Tox21_113597_1; AC-1539; BCP9000345; CCG-264772; CS-0116; DB06626; KS-1448; NSC 757441; NSC-799341; Benzamide, N-methyl-2-((3-((E)-2-(2-pyridinyl)ethenyl)-1H-indazol-6-yl)thio)-; NCGC00241108-04; NCGC00241108-06; HY-10065; SMR002530046; AM20090673; SW219464-1; D03218; AB01274739-01; AB01274739_02; 460A850; SR-01000941566; J-502064; Q-200662; Q4830631; SR-01000941566-1; BRD-K29905972-001-01-4; BRD-K29905972-001-02-2; Benzamide, N-methyl-2-((3-((1E)-2-(2-pyridinyl)ethenyl)-1H-indazo)-6-yl)thio)-; Benzamide, N-methyl-2-[[3-[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]-; N-Methyl-[[3[(1E)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]-benzamide; N-methyl-2-({3-[(E)-2-pyridin-2-ylethenyl]-2H-indazol-6-yl}sulfanyl)benzamide; N-METHYL-2-(3-((E)-2-PYRIDIN-2-YL-VINYL)-1H-INDAZOL-6-YLSULFANYL)-BENZAMIDE
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
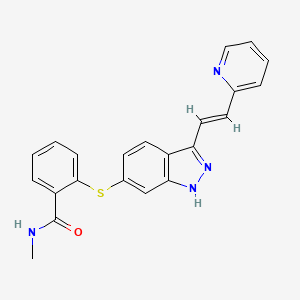
|
||||
| Target | Vascular endothelial growth factor receptor 2 (KDR) | VGFR2_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C22H18N4OS
|
||||
| IsoSMILES |
CNC(=O)C1=CC=CC=C1SC2=CC3=C(C=C2)C(=NN3)/C=C/C4=CC=CC=N4
|
||||
| InChI |
1S/C22H18N4OS/c1-23-22(27)18-7-2-3-8-21(18)28-16-10-11-17-19(25-26-20(17)14-16)12-9-15-6-4-5-13-24-15/h2-14H,1H3,(H,23,27)(H,25,26)/b12-9+
|
||||
| InChIKey |
RITAVMQDGBJQJZ-FMIVXFBMSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Tyrosine-protein kinase ABL1 (ABL1) | [2] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T315I (c.944C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.89 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.17 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
40
|
-
G
-
A
-
M
-
D
-
P
-
S
-
E
-
A
-
L
-
Q
50
|
-
R
-
P
-
V
-
A
-
S
-
D
-
F
-
E
-
P
-
Q
60
|
-
G
-
L
-
S
-
E
-
A
-
A
-
R
-
W
-
N
-
S
70
|
-
K
-
E
-
N
-
L
-
L
-
A
-
G
-
P
-
S
-
E
80
|
-
N
-
D
-
P
-
N
-
L
-
F
-
V
-
A
-
L
-
Y
90
|
-
D
-
F
-
V
-
A
-
S
-
G
-
D
-
N
-
T
-
L
100
|
-
S
-
I
-
T
-
K
-
G
-
E
-
K
-
L
-
R
-
V
110
|
-
L
-
G
-
Y
-
N
-
H
-
N
-
G
-
E
-
W
-
C
120
|
-
E
-
A
-
Q
-
T
-
K
-
N
-
G
-
Q
-
G
-
W
130
|
-
V
-
P
-
S
-
N
-
Y
M
I
A
T
S
P
V
V
N
N
140
|
S
S
L
L
E
E
K
K
H
H
S
S
W
W
Y
Y
H
H
G
G
150
|
P
P
V
V
S
S
R
R
N
N
A
A
A
A
E
E
Y
Y
L
L
160
|
L
L
S
S
S
S
G
G
I
I
N
N
G
G
S
S
F
F
L
L
170
|
V
V
R
R
E
E
S
S
E
E
S
S
S
S
P
P
G
G
Q
Q
180
|
R
R
S
S
I
I
S
S
L
L
R
R
Y
Y
E
E
G
G
R
R
190
|
V
V
Y
Y
H
H
Y
Y
R
R
I
I
N
N
T
T
A
A
S
S
200
|
D
D
G
G
K
K
L
L
Y
Y
V
V
S
S
S
S
E
E
S
S
210
|
R
R
F
F
N
N
T
T
L
L
A
A
E
E
L
L
V
V
H
H
220
|
H
H
H
H
S
S
T
T
V
V
A
A
D
D
G
G
L
L
I
I
230
|
T
T
T
T
L
L
H
H
Y
Y
P
P
A
A
P
P
K
K
R
R
240
|
N
N
K
K
P
P
T
T
V
V
Y
Y
G
G
V
V
S
S
P
P
250
|
N
N
Y
Y
D
D
K
K
W
W
E
E
M
M
E
E
R
R
T
T
260
|
D
D
I
I
T
T
M
M
K
K
H
H
K
K
L
L
G
G
G
G
270
|
G
G
Q
Q
Y
Y
G
G
E
E
V
V
Y
Y
E
E
G
G
V
V
280
|
W
W
K
K
K
K
Y
Y
S
S
L
L
T
T
V
V
A
A
V
V
290
|
K
K
T
T
L
L
K
K
E
E
D
D
T
T
M
M
E
E
V
V
300
|
E
E
E
E
F
F
L
L
K
K
E
E
A
A
A
A
V
V
M
M
310
|
K
K
E
E
I
I
K
K
H
H
P
P
N
N
L
L
V
V
Q
Q
320
|
L
L
L
L
G
G
V
V
C
C
T
T
R
R
E
E
P
P
P
P
330
|
F
F
Y
Y
I
I
I
I
T
I
E
E
F
F
M
M
T
T
Y
Y
340
|
G
G
N
N
L
L
L
L
D
D
Y
Y
L
L
R
R
E
E
C
C
350
|
N
N
R
R
Q
Q
E
E
V
V
N
N
A
A
V
V
V
V
L
L
360
|
L
L
Y
Y
M
M
A
A
T
T
Q
Q
I
I
S
S
S
S
A
A
370
|
M
M
E
E
Y
Y
L
L
E
E
K
K
K
K
N
N
F
F
I
I
380
|
H
H
R
R
D
N
L
L
A
A
A
A
R
R
N
N
C
C
L
L
390
|
V
V
G
G
E
E
N
N
H
H
L
L
V
V
K
K
V
V
A
A
400
|
D
D
F
F
G
G
L
L
S
S
R
R
L
L
M
M
T
T
G
G
410
|
D
D
T
T
Y
Y
T
T
A
A
H
H
A
A
G
G
A
A
K
K
420
|
F
F
P
P
I
I
K
K
W
W
T
T
A
A
P
P
E
E
S
S
430
|
L
L
A
A
Y
Y
N
N
K
K
F
F
S
S
I
I
K
K
S
S
440
|
D
D
V
V
W
W
A
A
F
F
G
G
V
V
L
L
L
L
W
W
450
|
E
E
I
I
A
A
T
T
Y
Y
G
G
M
M
S
S
P
P
Y
Y
460
|
P
P
G
G
I
I
D
D
L
L
S
S
Q
Q
V
V
Y
Y
E
E
470
|
L
L
L
L
E
E
K
K
D
D
Y
Y
R
R
M
M
E
E
R
R
480
|
P
P
E
E
G
G
C
C
P
P
E
E
K
K
V
V
Y
Y
E
E
490
|
L
L
M
M
R
R
A
A
C
C
W
W
Q
Q
W
W
N
N
P
P
500
|
S
S
D
D
R
R
P
P
S
S
F
F
A
A
E
E
I
I
H
H
510
|
Q
Q
A
A
F
F
E
E
T
T
M
M
F
F
Q
Q
E
E
S
S
520
|
S
S
I
I
S
S
D
D
E
E
V
V
E
E
K
K
E
E
L
L
530
|
G
G
K
K
Q
Q
G
G
V
V
L
-
E
-
H
-
H
-
H
-
540
|
H
-
H
-
H
-
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | Bone marrow | N.A. | |||||||||||
| Mechanism Description | The missense mutation p.T315I (c.944C>T) in gene ABL1 cause the sensitivity of Axitinib by aberration of the drug's therapeutic target | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.T670I (c.2009C>T) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.25 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.40 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
G
-
S
550
|
-
M
-
P
-
M
-
Y
-
E
-
V
-
Q
-
W
-
K
-
V
560
|
-
V
-
E
-
E
-
S
-
N
G
G
N
N
N
N
Y
Y
V
S
570
|
Y
Y
I
I
D
D
P
P
T
T
Q
Q
L
L
P
P
Y
Y
D
D
580
|
H
H
K
K
W
W
E
E
F
F
P
P
R
R
N
N
R
R
L
L
590
|
S
S
F
F
G
G
K
K
T
T
L
L
G
G
A
A
G
G
A
A
600
|
F
F
G
G
K
K
V
V
V
V
E
E
A
A
T
T
A
A
Y
Q
610
|
G
G
L
L
I
I
K
K
S
S
D
D
A
A
A
A
M
M
T
T
620
|
V
V
A
A
V
V
K
K
M
M
L
L
K
K
P
P
S
S
A
A
630
|
H
H
L
S
T
T
E
E
R
R
E
E
A
A
L
L
M
M
S
S
640
|
E
E
L
L
K
K
V
V
L
L
S
S
Y
Y
L
L
G
G
N
N
650
|
H
H
M
E
N
N
I
I
V
V
N
N
L
L
L
L
G
G
A
A
660
|
C
C
T
T
I
H
G
G
G
G
P
P
T
T
L
L
V
V
I
I
670
|
T
I
E
E
Y
Y
C
C
C
C
Y
Y
G
G
D
D
L
L
L
L
680
|
N
N
F
F
L
L
R
R
R
R
K
K
R
R
D
D
S
E
F
F
690
|
I
V
C
P
S
Y
K
K
T
-
-
-
-
-
-
-
-
-
-
-
700
|
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
710
|
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
720
|
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
730
|
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
740
|
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
750
|
-
-
-
-
-
-
S
-
P
V
A
A
I
P
-
E
-
D
-
L
760
|
-
Y
E
K
L
D
A
F
L
L
D
T
L
L
E
E
D
H
L
L
770
|
L
L
S
S
F
F
S
S
Y
Y
Q
Q
V
V
A
A
K
K
G
G
780
|
M
M
A
A
F
F
L
L
A
A
S
S
K
K
N
N
C
C
I
I
790
|
H
H
R
R
D
D
L
L
A
A
A
A
R
R
N
N
I
I
L
L
800
|
L
L
T
T
H
H
G
G
R
N
I
I
T
T
K
K
I
I
C
C
810
|
D
D
F
F
G
G
L
L
A
A
R
R
D
D
I
I
K
K
N
N
820
|
D
D
S
S
N
N
Y
Y
V
V
V
D
K
K
G
G
N
N
A
A
830
|
R
R
L
L
P
P
V
V
K
K
W
W
M
M
A
A
P
P
E
E
840
|
S
S
I
I
F
F
N
N
C
S
V
V
Y
Y
T
T
F
F
E
E
850
|
S
S
D
D
V
V
W
W
S
S
Y
Y
G
G
I
I
F
F
L
L
860
|
W
W
E
E
L
L
F
F
S
S
L
L
G
G
S
S
S
S
P
P
870
|
Y
Y
P
P
G
G
M
M
P
P
V
V
D
D
S
S
K
K
F
F
880
|
Y
Y
K
K
M
M
I
I
K
K
E
E
G
G
F
F
R
R
M
M
890
|
L
S
S
S
P
P
E
E
H
Y
A
A
P
P
A
A
E
E
M
M
900
|
Y
Y
D
D
I
I
M
M
K
K
T
T
C
C
W
W
D
D
A
A
910
|
D
D
P
P
L
D
K
K
R
R
P
P
T
T
F
F
K
K
Q
Q
920
|
I
I
V
V
Q
Q
L
D
I
I
E
E
K
K
Q
Q
I
I
S
S
930
|
-
E
-
S
-
T
-
N
-
H
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V559D (c.1676T>A) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V559A (c.1676T>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V559G (c.1676T>G) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.L576P (c.1727T>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.V654A (c.1961T>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | ||||||||||||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | ||||||||||||
| Molecule Alteration | Missense mutation | p.A829P (c.2485G>C) |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 | |||||||||
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | ||||||||||
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | ||||||||||
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | ||||||||||
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | ||||||||||
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | |||||||||||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | ||||||||||||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | ||||||||||||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Molecule Alteration | Missense mutation | p.V559D (c.1676T>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | |
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | |
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | |
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Molecule Alteration | IF-deletion | p.V560_Y578del19 (c.1679_1735del57) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | |
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | |
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | |
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
| Key Molecule: Mast/stem cell growth factor receptor Kit (KIT) | [3] | |||
| Sensitive Disease | Gastrointestinal stromal tumor [ICD-11: 2B5B.0] | |||
| Molecule Alteration | Missense mutation | p.K642E (c.1924A>G) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | GIST-T1 cells | Gastric | Homo sapiens (Human) | CVCL_4976 |
| Ba/F3 cells | Colon | Homo sapiens (Human) | CVCL_0161 | |
| GIST-882 cells | Gastric | Homo sapiens (Human) | CVCL_7044 | |
| GIST-5R cells | Gastric | Homo sapiens (Human) | CVCL_A9M9 | |
| GIST-48B cells | Gastric | Homo sapiens (Human) | CVCL_M441 | |
| In Vivo Model | Female BALB/c-nu/nu mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Whole transcriptome shotgun sequencing assay | |||
| Experiment for Drug Resistance |
CellTiter-Glo assay; IC50 assay | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
