Drug Information
Drug (ID: DG01165) and It's Reported Resistant Information
| Name |
Guggulsterone
|
||||
|---|---|---|---|---|---|
| Synonyms |
(Z)-Guggulsterone; Z-Guggulsterone; Guggulsterone; 39025-23-5; 95975-55-6; Guggulsterones Z; Guggulsterone E&Z; GUGGULSTERONE Z; Cis-Guggulsterone; UNII-6CST3U34GN; 6CST3U34GN; (Z)-Pregna-4,17(20)-diene-3,16-dione; Pregna-4,17(20)-diene-3,16-dione; (8R,9S,10R,13S,14S,17Z)-17-ethylidene-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15-decahydrocyclopenta[a]phenanthrene-3,16-dione; (17Z)-Pregna-4,17(20)-diene-3,16-dione; Gugulsterone; Guggulsterone, (Z)-; Pregna-4,17(20)-diene-3,16-dione, (17Z)-; Guggulsterones E&Z; GS; (E&Z)-Guggulsterone; DSSTox_CID_13539; DSSTox_RID_79083; DSSTox_GSID_33539; SCHEMBL141657; CHEMBL410683; DTXSID1033539; BDBM21725; CHEBI:135338; BCP18087; Tox21_202518; MFCD01310757; AKOS015963432; ZINC118912814; (Z)-Guggulsterone, analytical standard; AC-6215; CCG-267610; NCGC00091910-01; NCGC00260067-01; AC-28813; AS-79083; (17Z)-pregna-4,17-diene-3,16-dione; 4,17(20)-trans-Pregnadiene-3,16-dione; CAS-39025-23-5; HY-107738; CS-0029421; S3792; V2228; (Z)-Guggulsterone, >=89% (HPLC), powder; 2-(2-Aminothiazol-3(2H)-yl)-1-phenylethanone; 975G556; BRD-K26674531-001-01-3; Q27264514; UNII-A4PW148END component WDXRGPWQVHZTQJ-OSJVMJFVSA-N; Guggulsterone Z, United States Pharmacopeia (USP) Reference Standard; (1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-5,13-dione
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
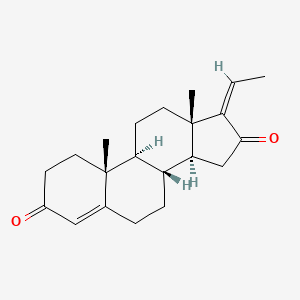
|
||||
| Target | Farnesoid X-activated receptor (FXR) | NR1H4_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C21H28O2
|
||||
| IsoSMILES |
C/C=C/1\\C(=O)C[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C
|
||||
| InChI |
1S/C21H28O2/c1-4-16-19(23)12-18-15-6-5-13-11-14(22)7-9-20(13,2)17(15)8-10-21(16,18)3/h4,11,15,17-18H,5-10,12H2,1-3H3/b16-4+/t15-,17+,18+,20+,21-/m1/s1
|
||||
| InChIKey |
WDXRGPWQVHZTQJ-OSJVMJFVSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Sensitive Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MDA PCa 2b cells | Prostate | Homo sapiens (Human) | CVCL_4748 |
| Experiment for Molecule Alteration |
western blot; flow cytometry | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Guggulsterone is a novel and potent MDR reversal agent with the potential to be an adjunctive agent for tumor chemotherapy. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
