Drug Information
Drug (ID: DG01148) and It's Reported Resistant Information
| Name |
Nitrofurazone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Nitrofurazone; Nitrofural; 59-87-0; Furacilin; Furacin; Furacillin; Actin-N; Aldomycin; Furacine; Furaldon; Nifuzon; 5-Nitro-2-furaldehyde semicarbazone; Babrocid; Chemofuran; Furacinetten; Furacoccid; Furacycline; Furaplast; Furaziline; Furazone; Mastofuran; Monafuracin; Nitrofurazan; Nitrozone; Otofuran; Alfucin; Amifur; Furesol; Mammex; Furazol W; 5-Nitrofurfural semicarbazone; Becafurazone; Biofuracina; Dermofural; Furametral; Furaseptyl; Furatsilin; Fuvacillin; Monafuracis; Monofuracin; Nitrofurol; Biofurea; Cocafurin; Coxistat; Dynazone; Eldezol; Fedacin; Flavazone; Fracine; Furacort; Furaderm; Furagent; Furalone; Furaskin; Furazin; Furazina; Furazyme; Furfurin; Furosem; Hemofuran; Ibiofural; Nifucin; Nifurid; Otofural; Sanfuran; Vabrocid; Vadrocid; Yatrocin; Chixin; Nefco; 5-Nitrofurazone; Furan-Ofteno; Spray-Dermis; Spray-Foral; Furacin-Hc; Nitrofuralum; Eldezol F-6; Furacilinum; Nitrofurane; Furacin-E; Nitrofuraldehyde semicarbazone; (5-Nitro-2-furfurylidenamino)urea; 5-Nitrofuraldehyde semicarbazide; Usaf ea-4; Rivafurazon; Fura-septin; Veterinary nitrofurazone; Nitrofuran (bactericide); NF-7; 6-Nitrofuraldehyde semicarbazide; 5-Nitro-2-furfural semicarbazone; 1-(5-Nitro-2-furfurylidene)semicarbazide; NSC-2100; 5-Nitrofuran-2-aldehyde semicarbazone; 5-Nitro-2-furfuraldehyde semicarbazone; NCI-C56064; 5-Nitro-2-furancarboxaldehyde semicarbazone; NFZ; UNII-X8XI70B5Z6; Nitrofural [INN]; Semikarbazon 5-nitrofurfuralu; Hydrazinecarboxamide, 2-[(5-nitro-2-furanyl)methylene]-; MFCD00003225; U-6421; 2-Furaldehyde, 5-nitro-, semicarbazone; 5-nitrofuran-2-carbaldehyde semicarbazone; 2-[(5-Nitro-2-furanyl)methylene]-hydrazinecarboxamide; X8XI70B5Z6; NFS; 2-Furancarboxaldehyde, 5-nitro-, semicarbazone; NSC1602; NSC2100; component of Furea; (5-Nitro-2-furfurylideneamino)urea; Nitrofural (INN); NSC-1602; component of Furadex; component of Furacort; Hydrazinecarboxamide, 2-((5-nitro-2-furanyl)methylene)-; NCGC00090686-04; NCGC00090686-07; Nitrofurazonum; DSSTox_CID_944; [(E)-(5-nitrofuran-2-yl)methylideneamino]urea; DSSTox_RID_75881; DSSTox_GSID_20944; 2-((5-Nitrofuran-2-yl)methylene)hydrazinecarboxamide; 2-[(5-Nitro-2-furanyl)methylene]hydrazinecarboxamide; WLN: T5OJ BNW E1UNMVZ; (2E)-2-[(5-nitrofuran-2-yl)methylidene]hydrazinecarboxamide; Dymazone; Furalcyn; Acutol; 2-Furancarboxaldehyde, semicarbazone; Rivopon-5; SR-05000002027; 2-((5-Nitro-2-furanyl)methylene)hydrazinecarboxamide; Nitrofuralum [INN-Latin]; CHEBI:44368; CCRIS 1195; Nitrofurazone [USP:INN:BAN]; Nfz mix; HSDB 3136; CAS-59-87-0; NSC 1602; NSC 2100; Prestwick_806; 2-[(5-nitro-2-furyl)methylene]hydrazinecarboxamide; EINECS 200-443-1; Furacin (TN); 2((5-Nitro-2-furanyl)methylene)hydrazinecarboxamide; Nitrofurazone (USP); Semikarbazon 5-nitrofurfuralu [Polish]; BRN 0086403; AI3-17333; [(E)-(5-nitro-2-furyl)methyleneamino]urea; Prestwick2_000492; Prestwick3_000492; Spectrum5_001160; Nitrofurazone (Nitrofural); CHEMBL869; SCHEMBL25416; SCHEMBL25417; BSPBio_000383; BSPBio_002075; MLS002153843; SPECTRUM1500434; BPBio1_000423; component of Furea (Salt/Mix); component of Furadex (Salt/Mix); HMS502G20; (E)-2-((5-nitrofuran-2-yl)methylene)hydrazine-1-carboxamide; HMS1569D05; HMS1920B04; HMS2091J04; HMS2096D05; HMS3713D05; Pharmakon1600-01500434; HY-B0226; ZINC4802968; Tox21_110997; Tox21_202988; Tox21_400035; BDBM50420350; CCG-39642; NSC757244; s1644; STK741625; 1-(5-Nitrofurfurylidene)semicarbazide; 5-Nitro-2-furaldehyde, semicarbazone; 5-Nitro-2-furfurylidene semicarbazone; AKOS000304771; Tox21_110997_1; DB00336; LS41202; MCULE-5485227436; NSC-757244; IDI1_000778; NCGC00090686-01; NCGC00090686-02; NCGC00090686-03; NCGC00090686-05; NCGC00090686-06; NCGC00090686-08; NCGC00090686-11; NCGC00260533-01; 112574-44-4; AC-10331; BS-42205; H823; Semioxamazide, 1-(5-nitrofurfurylidene)-; SMR000059012; SBI-0051458.P003; AB00373885; N0200; C08042; D00862; AB00373885-04; AB00373885_05; AB00373885_06; Structure of 5-nitro-2-furaldehyde-semicarbazone; [(E)-[(5-nitrofuran-2-yl)methylidene]amino]urea; Q-201480; SR-05000002027-1; SR-05000002027-3; BRD-K79092138-001-05-2; BRD-K79092138-001-06-0; 5-Nitro-2-furaldehyde semicarbazone, >=97.0% (HPLC); Nitrofural, European Pharmacopoeia (EP) Reference Standard; Nitrofurazone, United States Pharmacopeia (USP) Reference Standard; Nitrofural for peak identification, European Pharmacopoeia (EP) Reference Standard; Nitrofurazone solution, 100 mug/mL in acetonitrile, VETRANAL(TM), analytical standard; Nitrofurazone, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
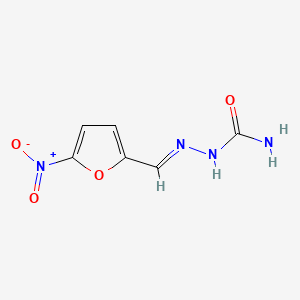
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Bacterial 30S ribosomal RNA (Bact 30S rRNA) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C6H6N4O4
|
||||
| IsoSMILES |
C1=C(OC(=C1)[N+](=O)[O-])/C=N/NC(=O)N
|
||||
| InChI |
1S/C6H6N4O4/c7-6(11)9-8-3-4-1-2-5(14-4)10(12)13/h1-3H,(H3,7,9,11)/b8-3+
|
||||
| InChIKey |
IAIWVQXQOWNYOU-FPYGCLRLSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Oxygen-insensitive NAD(P)H nitroreductase (NFSB) | [1] | |||
| Resistant Disease | Escherichia coli infection [ICD-11: 1A03.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | THP1 cells | Pleural effusion | Homo sapiens (Human) | CVCL_0006 |
| Mechanism Description | Escherichia coli contains at least two enzymes which reduce nitrofurazone and other nitrofuran derivatives. One of these enzymes is lacking in some nitrofurazone-resistant mutant strains. We now report that there are three separable nitrofuran reductases in this organism: reductase I (mol. wt. approximately 50 000, insensitive to O2), reductase IIa (mol. wt. approximately 120 000, inhibited by oxygen), reductase IIb (mol. wt. approximately 700 000, inhibited by O2). Unstable metabolites formed during the reduction of nitrofurazone by preparations containing reductases IIa and IIb produce breaks in DNA in vitro. In vivo experiments with nitrofurazone-resistant strains, which lack reductase II but contain reductases IIa and IIb, demonstrated that lethality, mutation, and DNA breakage are all greatly increased when cultures are incubated under anaerobic conditions, i.e., conditions such that reductase II is active. These results provide further evidence for the importance of reductive activation of nitrofurazone. | |||
| Key Molecule: Nitrofurazone-reductase IIa (NFR2A) | [1] | |||
| Resistant Disease | Escherichia coli infection [ICD-11: 1A03.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | THP1 cells | Pleural effusion | Homo sapiens (Human) | CVCL_0006 |
| Mechanism Description | Escherichia coli contains at least two enzymes which reduce nitrofurazone and other nitrofuran derivatives. One of these enzymes is lacking in some nitrofurazone-resistant mutant strains. We now report that there are three separable nitrofuran reductases in this organism: reductase I (mol. wt. approximately 50 000, insensitive to O2), reductase IIa (mol. wt. approximately 120 000, inhibited by oxygen), reductase IIb (mol. wt. approximately 700 000, inhibited by O2). Unstable metabolites formed during the reduction of nitrofurazone by preparations containing reductases IIa and IIb produce breaks in DNA in vitro. In vivo experiments with nitrofurazone-resistant strains, which lack reductase II but contain reductases IIa and IIb, demonstrated that lethality, mutation, and DNA breakage are all greatly increased when cultures are incubated under anaerobic conditions, i.e., conditions such that reductase II is active. These results provide further evidence for the importance of reductive activation of nitrofurazone. | |||
| Key Molecule: Nitrofurazone-reductase IIb (NFR2B) | [1] | |||
| Resistant Disease | Escherichia coli infection [ICD-11: 1A03.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | THP1 cells | Pleural effusion | Homo sapiens (Human) | CVCL_0006 |
| Mechanism Description | Escherichia coli contains at least two enzymes which reduce nitrofurazone and other nitrofuran derivatives. One of these enzymes is lacking in some nitrofurazone-resistant mutant strains. We now report that there are three separable nitrofuran reductases in this organism: reductase I (mol. wt. approximately 50 000, insensitive to O2), reductase IIa (mol. wt. approximately 120 000, inhibited by oxygen), reductase IIb (mol. wt. approximately 700 000, inhibited by O2). Unstable metabolites formed during the reduction of nitrofurazone by preparations containing reductases IIa and IIb produce breaks in DNA in vitro. In vivo experiments with nitrofurazone-resistant strains, which lack reductase II but contain reductases IIa and IIb, demonstrated that lethality, mutation, and DNA breakage are all greatly increased when cultures are incubated under anaerobic conditions, i.e., conditions such that reductase II is active. These results provide further evidence for the importance of reductive activation of nitrofurazone. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
