Drug Information
Drug (ID: DG00999) and It's Reported Resistant Information
| Name |
Desloratadine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Desloratadine; 100643-71-8; Clarinex; Descarboethoxyloratadine; Desloratidine; Neoclarityn; Aerius; Azomyr; Sch-34117; Denosin; Opulis; Allex; Sch 34117; 8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine; Descarboethoxyoratidine; MFCD00871949; UNII-FVF865388R; CHEMBL1172; Loratadine related compound a; C19H19ClN2; 8-chloro-11-(piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine; 8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H-benzo(5,6)cyclohepta(1,2-b)pyridine; MLS000559042; CHEBI:291342; FVF865388R; 5H-Benzo[5,6]cyclohepta[1,2-b]pyridine, 8-chloro-6,11-dihydro-11-(4-piperidinylidene)-; NSC-759824; NCGC00159325-02; SMR000149358; Clarinex RediTabs; 8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine; DSSTox_CID_24196; DSSTox_RID_80112; DSSTox_GSID_44196; Desalex; 5H-Benzo(5,6)cyclohepta(1,2-b)pyridine, 8-chloro-6,11-dihydro-11-(4-piperidinylidene)-; 8-chloro-11-piperidin-4-ylidene-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridine; 8-chloro-6,11-dihydro-11-(4-piperdinylidene)- 5H-benzo[5,6]cyclohepta[1,2-b]pyridine; Clarinex (TN); 13-chloro-2-(piperidin-4-ylidene)-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaene; Desloratadine Actavis; CAS-100643-71-8; SR-01000668962; Dasselta; Desloratadine [USAN:INN:BAN]; Desloratadine teva; Clarinex(R); 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta(1,2-b]pyridin-11-ylidene)-piperidine; 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine; 4-{8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene}-piperidine; 4-{8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene}piperidine; Desloratadine-[d7]; Desloratadine- Bio-X; MK-4117; CPD000149358; Opera_ID_1891; SCHEMBL4425; MLS000759406; MLS001201801; MLS001424247; Sch34117; Desloratadine (JAN/USP/INN); GTPL7157; ZINC1261; DTXSID1044196; Loratadine related compound a rs; HMS2052H05; HMS2090C06; HMS2093F19; HMS3394H05; HMS3652O15; HMS3715J15; HMS3885C18; Pharmakon1600-01505393; ALBB-027276; BCP02340; HY-B0539; Tox21_111574; BBL010777; BDBM50073179; NSC675447; NSC759824; s4012; STK586537; AKOS000280921; Tox21_111574_1; AC-1279; CCG-101162; DB00967; KS-1048; MCULE-2975958622; NC00412; NSC 675447; NSC 759824; NSC-675447; SB17503; Desloratadine, powder, >=98% (HPLC); NCGC00159325-03; NCGC00159325-04; NCGC00159325-05; 13-chloro-2-piperidin-4-ylidene-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaene; BD164361; Loratadine EP Impurity D (Desloratadine); SBI-0206828.P001; D3787; FT-0602522; FT-0666048; SW197792-3; A19515; D03693; H11943; J10309; AB00456701-11; AB00456701-13; AB00456701_14; AB00456701_15; 643D718; L001025; Q418060; Q-200936; SR-01000668962-4; SR-01000668962-5; SR-01000668962-8; BRD-K82357231-001-13-4; Desloratadine, European Pharmacopoeia (EP) Reference Standard; Desloratadine, United States Pharmacopeia (USP) Reference Standard; 4-(8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)piperidine; 4-(8-chloro-5,6-dihydro-11H-benzo-[5,6]cyclohepta(1,2-b]pyridin-11-ylidene)-piperidine; 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta [1,2-b]pyridin-11-ylidene) piperidine; 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene) piperidine; 8-chloro-11-(4-piperidinylidene)-6,11-dihydro-5H- benzo[4,5]cyclohepta[2,1-b]pyridine; 8-chloro-11-(4-piperidyliden)-6,11-dihydro-5H-benzo[5,6]cyclohepta [1,2-b]pyridine; 8-chloro-11-(4-piperidyliden)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine; 8-chloro-11-(4-piperidylidene)-6,11-dihydro-5h-benzo[5.6]cyclohepta[1,2-b]pyridine; 8-Chloro-6,11-dihydro-11-(4-piperidinylidene)-5H- benzo[5,6]cyclohepta[1,2,b]pyridine; 8-chloro-6,11-dihydro-11-(4-piperidylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine; Desloratadine for system suitability, European Pharmacopoeia (EP) Reference Standard; Desloratidine, Pharmaceutical Secondary Standard; Certified Reference Material; Loratadine Related Compound A, United States Pharmacopeia (USP) Reference Standard; 13-chloro-2-(piperidin-4-ylidene)-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,12,14-hexaene; 8-CHLORO-11-(4-PIPERIDYLIDENE)-6,11-DIHYDRO-5H-BENZO[5,6]CYCLOHEPTA[1,2-b]PYRIDINE; 8-Chloro-11-(piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine (Descarboethoxyloratadine; Desloratadine)
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
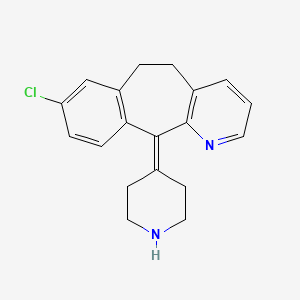
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(1 diseases)
[1]
|
||||
| Target | Histamine H1 receptor (H1R) | HRH1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C19H19ClN2
|
||||
| IsoSMILES |
C1CC2=C(C=CC(=C2)Cl)C(=C3CCNCC3)C4=C1C=CC=N4
|
||||
| InChI |
1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2
|
||||
| InChIKey |
JAUOIFJMECXRGI-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Protein kinase C alpha (PRKCA) | [1] | |||
| Resistant Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | ATCC 293T cells | Fetal kidney | Homo sapiens (Human) | CVCL_0063 |
| Experiment for Molecule Alteration |
Resazurin assay | |||
| Mechanism Description | HMOX1, PRKCA, and NEIL2 contribute to anthracycline resistance within the more complex MDR phenotype, while P-glycoprotein/ABCB1 overexpression causes not only anthracycline resistance but also resistance to other anticancer drug classes. Conivaptan has the potential to be used as the dual inhibitor of HMOX1 and PRKCA, whereas bexarotene has the potential as an HMOX1 inhibitor and desloratadine as a PRKCA inhibitor. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
