Drug Information
Drug (ID: DG00928) and It's Reported Resistant Information
| Name |
Imiquimod
|
||||
|---|---|---|---|---|---|
| Synonyms |
IMIQUIMOD; 99011-02-6; Aldara; Zyclara; 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine; 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine; Beselna; 4-Amino-1-isobutyl-1H-imidazo[4,5-c]quinoline; R 837; 1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine; 4-Amino-1-isobutyl-1H-imidazo(4,5-c)quinoline; R-837; 9050-31-1; S-26308; C14H16N4; UNII-P1QW714R7M; MFCD00866946; CHEMBL1282; 1-isobutylimidazo[4,5-c]quinolin-4-amine; P1QW714R7M; 1H-Imidazo[4,5-c]quinolin-4-amine, 1-(2-methylpropyl)-; CHEBI:36704; S26308; NSC-369100; NSC-759651; 1H-Imidazo(4,5-c)quinolin-4-amine, 1-(2-methylpropyl)-; NCGC00070736-02; Zartra; Imiquimod acetate; DSSTox_CID_21047; DSSTox_RID_79617; DSSTox_GSID_41047; Aldara (TN); CAS-99011-02-6; S 26308; SR-01000611320; Imiquimodum; Imiquimod [USAN:INN:BAN]; Vyloma; MTD-39; 1-(2-Methylpropyl)-1H-imidazole[4,5-c]quinoline-4-amine; HSDB 8129; TMX 101; TMX-101; Aldara; ; ; Beselna; Imiquimod,(S); Imiquimod- Bio-X; 6T0; Imiquimod - Aldara; Zyclara (TN); DZ-2636; (non-labelled)Imiquimod-d9; Imiquimod (JAN/USP/INN); SCHEMBL26136; MLS000083577; BIDD:GT0859; GTPL5003; DTXSID7041047; AOB6939; HMS2090M14; HMS2232G07; HMS3373B13; HMS3715N19; HMS3747A13; Pharmakon1600-01502351; BCP05151; HY-B0180; Tox21_110985; AC-529; BBL010772; BDBM50240849; NSC369100; NSC759651; NSC811538; s1211; STK583860; ZINC19632912; Imiquimod - CAS 99011-02-6; Imiquimod, >=98% (HPLC), solid; AKOS005507352; Tox21_110985_1; 1H-Imidazo[4, 1-(2-methylpropyl)-; CCG-208015; CS-2058; DB00724; KS-5218; MCULE-9421195760; NSC 369100; NSC 741062; NSC 759651; NSC-811538; YH44175; (Hydroxypropyl)methyl cellulose phthalate; Imiquimod 100 microg/mL in Acetonitrile; NCGC00070736-03; NCGC00070736-04; BI164576; SMR000048307; SY017571; FT-0602727; I0747; D02500; J10325; 1-isobutyl-1H-imidazo [4,5-c]quinolin-4-amine; 1-isobutyl-1H-imidazo[4,5-c]quinoline-4-amine; AB00399298-05; AB00399298-06; AB00399298-07; AB00399298_08; AB00399298_09; 011I026; 1-Isobutyl-1H-imidazo[4,5-c]quinolin-4-ylamine; A845945; Q423417; 1-(2-methylpropyl)-4-imidazo[4,5-c]quinolinamine; SR-01000611320-2; SR-01000611320-3; BRD-K26657438-001-01-2; BRD-K26657438-001-13-7; 1-(2-methylpropyl)-1Himidazo[4,5-c]quinolin-4-amine; 1-(2-methylpropyl)-1H-imidazo[4,5-c]-quinolin-4-amine; Imiquimod, United States Pharmacopeia (USP) Reference Standard
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
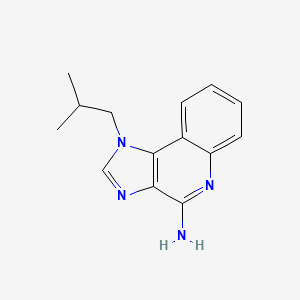
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Discovered by Cell Line Test for This Drug
(4 diseases)
[2]
[1]
[1]
[1]
|
||||
| Target | Toll-like receptor 7 (TLR7) | TLR7_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C14H16N4
|
||||
| IsoSMILES |
CC(C)CN1C=NC2=C1C3=CC=CC=C3N=C2N
|
||||
| InChI |
1S/C14H16N4/c1-9(2)7-18-8-16-12-13(18)10-5-3-4-6-11(10)17-14(12)15/h3-6,8-9H,7H2,1-2H3,(H2,15,17)
|
||||
| InChIKey |
DOUYETYNHWVLEO-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Interleukin-6 (IL6) | [2] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | TLR7 signaling pathway | Regulation | N.A. | |
| MAPK signaling pathway | Activation | hsa04010 | ||
| PI3K signaling pathway | Regulation | N.A. | ||
| JAK signaling pathway | Regulation | N.A. | ||
| PI3K/Akt signaling pathway | Activation | hsa04151 | ||
| JAK/STAT3 signaling pathway | Activation | hsa04630 | ||
| In Vitro Model | BCC/KMC-1 cells | N.A. | Homo sapiens (Human) | N.A. |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| Experiment for Molecule Alteration |
Immunoblotting assay | |||
| Experiment for Drug Resistance |
Cellular assay; Mitochondrial ROS assay; Lipid ROS assay; Cell viability assay; DNA content assay; Mitochondrial oxygen consumption assay | |||
| Mechanism Description | In this study, we demonstrated that Mcl-1 overexpression induced resistance to IMQ-induced apoptosis and reduced both IMQ-induced ROS generation and oxidative stress in cancer cells. Mcl-1 overexpression maintained mitochondrial function and integrity and prevented mitophagy in IMQ-treated cancer cells. Furthermore, IL-6 protected against IMQ-induced apoptosis by increasing Mcl-1 expression and attenuating IMQ-induced mitochondrial fragmentation. Mcl-1 overexpression ameliorates IMQ-induced ROS generation and mitochondrial fragmentation, thereby increasing mitochondrial stability and ultimately attenuating IMQ-induced cell death. Investigating the roles of Mcl-1 in mitochondria is a potential strategy for cancer therapy development. | |||
| Key Molecule: Myeloid cell leukemia 1 (Mcl-1) | [2] | |||
| Resistant Disease | Gastric adenocarcinoma [ICD-11: 2B72.0] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | TLR7 signaling pathway | Regulation | N.A. | |
| MAPK signaling pathway | Activation | hsa04010 | ||
| PI3K signaling pathway | Regulation | N.A. | ||
| JAK signaling pathway | Regulation | N.A. | ||
| PI3K/Akt signaling pathway | Activation | hsa04151 | ||
| JAK/STAT3 signaling pathway | Activation | hsa04630 | ||
| In Vitro Model | BCC/KMC-1 cells | N.A. | Homo sapiens (Human) | N.A. |
| AGS cells | Gastric | Homo sapiens (Human) | CVCL_0139 | |
| Experiment for Molecule Alteration |
Immunoblotting assay | |||
| Experiment for Drug Resistance |
Cellular assay; Mitochondrial ROS assay; Lipid ROS assay; Cell viability assay; DNA content assay; Mitochondrial oxygen consumption assay | |||
| Mechanism Description | In this study, we demonstrated that Mcl-1 overexpression induced resistance to IMQ-induced apoptosis and reduced both IMQ-induced ROS generation and oxidative stress in cancer cells. Mcl-1 overexpression maintained mitochondrial function and integrity and prevented mitophagy in IMQ-treated cancer cells. Furthermore, IL-6 protected against IMQ-induced apoptosis by increasing Mcl-1 expression and attenuating IMQ-induced mitochondrial fragmentation. Mcl-1 overexpression ameliorates IMQ-induced ROS generation and mitochondrial fragmentation, thereby increasing mitochondrial stability and ultimately attenuating IMQ-induced cell death. Investigating the roles of Mcl-1 in mitochondria is a potential strategy for cancer therapy development. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Resistant Disease | Melanoma [ICD-11: 2C30.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Resazurin Cell Viability Assay | |||
| Mechanism Description | Imidazoquinolines IMQ, RSQ, and GDQ are substrates for P-gp and begins to elucidate differences in their trafficking in cancer cells as a consequence of acquired drug resistance. We believe this work that begins to examine imidazoquinoline trafficking will prove useful in the future rational design of immunotherapeutics with enhanced susceptibility to P-gp efflux that enable increased bioavailability, in MDR cancers. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | COLO205 cells | Colon | Homo sapiens (Human) | CVCL_F402 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Resazurin Cell Viability Assay | |||
| Mechanism Description | Imidazoquinolines IMQ, RSQ, and GDQ are substrates for P-gp and begins to elucidate differences in their trafficking in cancer cells as a consequence of acquired drug resistance. We believe this work that begins to examine imidazoquinoline trafficking will prove useful in the future rational design of immunotherapeutics with enhanced susceptibility to P-gp efflux that enable increased bioavailability, in MDR cancers. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | Hep3B cells | Liver | Homo sapiens (Human) | CVCL_0326 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
Resazurin Cell Viability Assay | |||
| Mechanism Description | Imidazoquinolines IMQ, RSQ, and GDQ are substrates for P-gp and begins to elucidate differences in their trafficking in cancer cells as a consequence of acquired drug resistance. We believe this work that begins to examine imidazoquinoline trafficking will prove useful in the future rational design of immunotherapeutics with enhanced susceptibility to P-gp efflux that enable increased bioavailability, in MDR cancers. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
