Drug Information
Drug (ID: DG00895) and It's Reported Resistant Information
| Name |
Danazol
|
||||
|---|---|---|---|---|---|
| Synonyms |
Danazol; Danocrine; 17230-88-5; Chronogyn; Winobanin; Danol; Cyclomen; Danazolum; Danzol; Ladogal; Danatrol; Danazol, USP; UNII-N29QWW3BUO; WIN-17757; WIN 17,757; Danazole; N29QWW3BUO; WIN 17757; Danovaol; CHEBI:4315; (1R,3aS,3bR,10aR,10bS,12aS)-1-ethynyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-cyclopenta[7,8]phenanthro[3,2-d]isoxazol-1-ol; Danokrin; NCGC00164400-01; Danogen; Pregna-2,4-dien-20-yno(2,3-d)isoxazol-17-ol, (17alpha)-; DSSTox_CID_2880; Danazolum [INN-Latin]; NSC 270916; DSSTox_RID_76772; DSSTox_GSID_22880; Bonzol; Danocrine (TN); SMR000058321; CCRIS 6747; EINECS 241-270-1; Vasaloc; 17-alpha-Pregn-4-en-20-yno(2,3-d)isoxazol-17-ol; Optina; Pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol, (17alpha)-; 17-alpha-2,4-Pregnadien-20-yno(2,3-d)isoxazol-17-ol; 17alpha-Pregna-2,4-dien-20-yno(2,3-d)isoxazol-17-ol; NSC270916; NSC-270916; Win 17, 757; Prestwick_150; CAS-17230-88-5; Danazol [USAN:USP:INN:BAN:JAN]; ethynyl(dimethyl)[ ]ol; 2,3-Isoxazolethisterone; Prestwick0_000105; Prestwick1_000105; Prestwick2_000105; Prestwick3_000105; CHEMBL1479; SCHEMBL21107; BSPBio_000090; (1S,2R,13R,14S,17R,18S)-17-ethynyl-2,18-dimethyl-7-oxa-6-azapentacyclo[11.7.0.0^{2,10}.0^{4,8}.0^{14,18}]icosa-4(8),5,9-trien-17-ol; MLS001066617; MLS001306473; Danazol (JP17/USP/INN); SPBio_002029; BPBio1_000100; GTPL6942; DTXSID2022880; 2,4,17alpha-Pregnadien-20-yno[2,3-d]-isoxa-zol-17-ol; Win-17,757; HMS1568E12; HMS2090A22; HMS2095E12; HMS2231M08; HMS3259M10; HMS3712E12; Pregna-2,4-dien-20-yno(2,3-d)isoxazol-17-ol,(17alpha)-; [1,2]oxazolo[4',5':2,3]-17alpha-pregn-4-en-20-yn-17-ol; 17.Alpha.-Pregna-2, {4-dien-20-yno[2,3-d]isoxazol-17-ol}; BCP11914; HY-B1029; ZINC3881958; Tox21_112114; Tox21_301940; BDBM50423541; MFCD00056838; s9506; AKOS015961192; Tox21_112114_1; AC-6836; CCG-220105; CS-4547; Danazol 100 microg/mL in Acetonitrile; DB01406; MCULE-5533294331; NC00557; NCGC00179665-01; NCGC00179665-02; NCGC00179665-04; NCGC00255335-01; (1R,3aS,3bR,10aR,10bS,12aS)-1-Ethynyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-cyclopenta[7,8]phenanthro[3,2-d][1,2]oxazol-1-ol; 1-Ethynyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-cyclopenta[7,8]phenanthro[3,2-d]isoxazol-1-ol; AS-13035; H066; C06938; D00289; 230D885; Q419652; SR-01000760722; SR-05000000445; SR-01000760722-2; SR-05000000445-2; W-107864; 17a-Pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol; BRD-K48970916-001-03-0; (17a)-Pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol; 17a-Pregna-2,4-dien-20-yne-[2,3-d]isoxazole-17b-ol; 1-(P-TOSYL)-3,4,4-TRIMETHYL-2-IMIDAZOLINIUMIODIDE; 17 alpha-pregna-2,4-dien-20-yno[2,3-D] isoxazol-17 beta-ol; Pregna-2, {4-dien-20-yno[2,3-d]isoxazol-17-ol,} (17.alpha.)-; Pregna-2,4-diene-20-yno[2,3-d ]isoxazol-17-ol, (17.alpha.); (1S,2R,13R,14S,17R,18S)-17-ethynyl-2,18-dimethyl-7-oxa-6-azapentacyclo[11.7.0.02,10.04,8.014,18]icosa-4(8),5,9-trien-17-ol; 1-ethynyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-10a,12a-dimethyl-1H-Cyclopenta[7,8]phenanthro[3,2-d]isoxazol-1-ol; 1H-Cyclopenta[7,8]phenanthro[3,2-d]isoxazole- pregna-2,4-dien-20-yno[2,3-d]isoxazol-17-ol deriv.
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
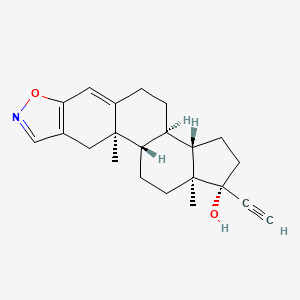
|
||||
| Target | Estrogen receptor (ESR) | ESR1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C22H27NO2
|
||||
| IsoSMILES |
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=CC5=C(C[C@]34C)C=NO5
|
||||
| InChI |
1S/C22H27NO2/c1-4-22(24)10-8-18-16-6-5-15-11-19-14(13-23-25-19)12-20(15,2)17(16)7-9-21(18,22)3/h1,11,13,16-18,24H,5-10,12H2,2-3H3/t16-,17+,18+,20+,21+,22+/m1/s1
|
||||
| InChIKey |
POZRVZJJTULAOH-LHZXLZLDSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Tyrosine-protein kinase JAK2 (JAK3) | [1] | |||
| Sensitive Disease | Primary myelofibrosis [ICD-11: 2A20.2] | |||
| Molecule Alteration | Function | Inhibition |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | In non-transplant candidates, conventional treatment for anemia includes androgens, prednisone, thalidomide, and danazol; for symptomatic splenomegaly, hydroxyurea and ruxolitinib; and for constitutional symptoms, ruxolitinib. Fedratinib, another JAK2 inhibitor, has now been FDA-approved for use in ruxolitinib failures. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
