Drug Information
Drug (ID: DG00659) and It's Reported Resistant Information
| Name |
Vardenafil
|
||||
|---|---|---|---|---|---|
| Synonyms |
Vardenafil; 224785-90-4; Levitra; Vivanza; UNII-UCE6F4125H; Levitra (TN); 2-{2-ETHOXY-5-[(4-ETHYLPIPERAZIN-1-YL)SULFONYL]PHENYL}-5-METHYL-7-PROPYLIMIDAZO[5,1-F][1,2,4]TRIAZIN-4(1H)-ONE; CHEMBL1520; UCE6F4125H; VDN; CHEBI:46295; BAY38-9456; BAY 38-9456; Vardenafil-d5; 224789-15-5; 2-(2-ethoxy-5-((4-ethylpiperazin-1-yl)sulfonyl)phenyl)-5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(1H)-one; 2-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonylphenyl]-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one; Vardenafil ODT; 2-(2-Ethoxy-5-((4-ethylpiperazin-1-yl)sulfonyl)phenyl)-5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(3H)-one; 2-{2-ethoxy-5-[(4-ethylpiperazin-1-yl)sulfonyl]phenyl}-5-methyl-7-propyl-1H,4H-imidazo[4,3-f][1,2,4]triazin-4-one; HSDB 7304; SR-05000001442; NCGC00167533-01; Vardenafil [USAN:INN:BAN]; Vardenafil (Vivanza); 1189685-70-8; 2-(2-Ethoxy-5-(4-ethylpiperazin-1-yl-1-sulfonyl)phenyl)-5-methyl-7-propyl-3H-imidazo(5,1-f)(1,2,4)triazin-4-one; Vardenafil (USAN/INN); EC 607-088-5; SCHEMBL5772; BSPBio_002392; 1-((3-(3,4-Dihydro-5-methyl-4-oxo-7-propylimidazo(5,1-f)-as-triazin-2-yl)-4-ethoxyphenyl)sulfonyl)-4-ethylpiperazine; GTPL7320; DTXSID3048318; BDBM14776; HMS2089K04; HMS3715F19; BCP02961; EX-A2806; HY-B0442; STK642629; ZINC18324776; AKOS005574038; AKOS015994765; AKOS022186043; BAY-389456; CCG-221149; DB00862; GS-6519; KS-5073; MCULE-5211071344; SB17349; NCGC00167533-03; 2-{2-ethoxy-5-[(4-ethylpiperazin-1-yl)sulfonyl]phenyl}-5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4-ol; Piperazine, 1-((3-(1,4-dihydro-5-methyl-4-oxo-7-propylimidazo(5,1-f)(1,2,4)triazin-2-yl)-4-ethoxyphenyl)sulfonyl)-4-ethyl-; BCP0726000254; SBI-0206836.P001; FT-0631204; FT-0675783; 85V904; D08668; AB00698215-08; AB01274740-01; AB01274740_02; AB01274740_03; AB01275440-01; Q424161; J-014730; SR-05000001442-1; SR-05000001442-2; BRD-K13926615-003-01-7; BRD-K13926615-003-02-5; Z2790753946; 2-(2-Ethoxy-5-((4-ethylpiperazin-1-yl)sulfonyl)phenyl)-5-methyl-7-propylimidazo(5,1-f)triazin-4(3H)-one; 2-(2-ethoxy-5-(4-ethylpiperazin-1-ylsulfonyl)phenyl)-5-methyl-7-propylimidazo[1,5-f][1,2,4]triazin-4(1H)-one; 2-[2-ethoxy-5-(4-ethyl-piperazine-1-sulphonyl)-phenyl]-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one; 2-[2-ethoxy-5-(4-ethyl-piperazine-1-sulphonyl)-phenyl]-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazine-4-one; 2-[2-ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-5-methyl-7-propyl-1H,4H-imidazo[4,3-f][1,2,4]triazin-4-one; 2-[2-ethoxy-5-[(4-ethyl-1-piperazinyl)sulfonyl]phenyl]-5-methyl-7-propyl-imidazo[5,1-f][1,2,4]triazin-4-one; 2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phenyl}-5-methyl-7-propyl-3H,4H-imidazo[1,5-a][1,2,4]triazin-4-one; citric acid;2-[2-ethoxy-5-(4-ethylpiperazino)sulfonyl-phenyl]-5-methyl-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-4-one
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
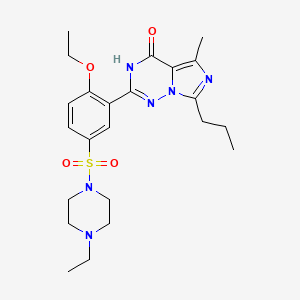
|
||||
| Target | Phosphodiesterase 5A (PDE5A) | PDE5A_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C23H32N6O4S
|
||||
| IsoSMILES |
CCCC1=NC(=C2N1N=C(NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)CC)OCC)C
|
||||
| InChI |
1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30)
|
||||
| InChIKey |
SECKRCOLJRRGGV-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| SJG 2 cells | Ora cavity | Homo sapiens (Human) | CVCL_WV26 | |
| ATCC 293T cells | Fetal kidney | Homo sapiens (Human) | CVCL_0063 | |
| Experiment for Molecule Alteration |
Western blot analysis; Immunofluorescence analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | Vardenafil reverses ABCB1-mediated MDR by directly blocking the drug efflux function of ABCB1. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
