Drug Information
Drug (ID: DG00641) and It's Reported Resistant Information
| Name |
Topiramate
|
||||
|---|---|---|---|---|---|
| Synonyms |
Topiramate; 97240-79-4; Topamax; Epitomax; Topamax Sprinkle; Tipiramate; Topiramatum; Topina; McN-4853; Tipiramato; Topiramato; Topimax; Topomax; McN 4853; C12H21NO8S; 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; RWJ-17021-000; RWJ-17021; 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamate; UNII-0H73WJJ391; USL255; USL-255; MFCD00865320; RWJ 17021; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate; CHEBI:63631; Tipiramate [French]; Tipiramato [Spanish]; 0H73WJJ391; ((3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4',5'-d]pyran-3a-yl)methyl sulfamate; Topiramatum [INN-Latin]; topiramatum [Latin]; Topiramato [INN-Spanish]; Epitoma; Topamac; DSSTox_CID_3688; DSSTox_RID_77148; DSSTox_GSID_23688; ((3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl)methyl sulfamate; [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.0^{2,6}]dodecan-6-yl]methyl sulfamate; Topiragen; Topax; Trokendi XR; Qudexy XR; SMR000466325; Topiramate (TPM); TOR; TPM; Topamax (TN); CAS-97240-79-4; BRN 5988957; Sincronil; Qudexy; HSDB 7531; Sulfamate 7; 3hku; 3lxe; USL 255; (-)-topiramate; Topiramate [USAN:USP:INN:BAN]; Topiramate solution; NCGC00095181-01; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyl-5,5a,8a,8b-tetrahydrodi[1,3]dioxolo[5,4-b:5',3'-d]pyran-3a-yl]methyl sulfamate; Topiramate- Bio-X; Trokendi xr (TN); KS-1122; Spectrum2_001128; beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate; CBChromo1_000352; BIDD:PXR0127; SCHEMBL34631; BSPBio_002306; Topiramate (JAN/USP/INN); Eprontia (liquid formulation); MLS000759431; MLS001424070; BIDD:GT0854; SPECTRUM1505801; SPBio_000995; CHEMBL220492; GTPL6849; DTXSID8023688; BDBM10887; HMS1922H06; HMS2051L09; HMS2093D20; HMS2232H21; HMS3414C15; HMS3678C15; HMS3715I12; HMS3884C17; Pharmakon1600-01505801; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate (non-preferred name); ACT09031; ALBB-022457; HY-B0122; Tox21_111472; Tox21_302401; 2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate; NSC759251; s1438; ZINC95616603; AKOS000424547; Topiramate 1.0 mg/ml in Acetonitrile; Topiramate, >=98% (HPLC), solid; Tox21_111472_1; CCG-100940; CS-1885; DB00273; MCULE-9403222429; NC00190; NSC 759251; NSC-759251; NCGC00178714-01; NCGC00178714-04; NCGC00178714-18; NCGC00255221-01; BT167048; SBI-0206907.P001; SW197570-3; C07502; D00537; F20536; AB00639961-06; AB00639961-08; AB00639961_09; AB00639961_10; 240T794; A900173; Q221174; SR-01000759409; W-60376; Q-201845; SR-01000759409-4; Z1522553470; 2,3:4,5-Di-O-isopropylidene-b-D-fructopyranose sulfamate; Topiramate, United States Pharmacopeia (USP) Reference Standard; 2,3:4,5-Bis-O-(1-methylethylidene)- -D-fructopyranose sulfamate; Topiramate, Pharmaceutical Secondary Standard; Certified Reference Material; Topiramate solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; [(1R,2S,6S,9R)-4,4,11,11-tetramethyl-3,5,7,10,12-pentaoxatricyclo[7.3.0.02,6]dodecan-6-yl]methyl sulfamate; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyl-5,5a,8a,8b-tetrahydrodi[1,3]dioxolo[4,5-a:5',3'-d]pyran-3a-yl]methyl sulfamate; 2,3:4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose 1-sulfamate;2,3:4,5-di-O-isopropylidene-beta-D-fructopyranose sulfamate; Topamax; Tracrium; Toiramate:
Click to Show/Hide
|
||||
| Indication |
In total 4 Indication(s)
|
||||
| Structure |
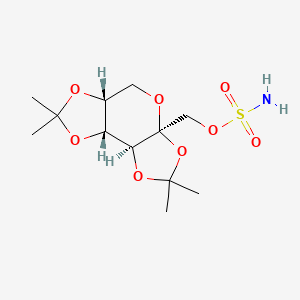
|
||||
| Target | Glutamate receptor ionotropic kainate 1 (GRIK1) | GRIK1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C12H21NO8S
|
||||
| IsoSMILES |
CC1(O[C@@H]2CO[C@@]3([C@H]([C@@H]2O1)OC(O3)(C)C)COS(=O)(=O)N)C
|
||||
| InChI |
1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1
|
||||
| InChIKey |
KJADKKWYZYXHBB-XBWDGYHZSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Potassium inwardly rectifying channel subfamily J member 10 (KCNJ10) | [1] | |||
| Sensitive Disease | Genetic generalized epilepsies [ICD-11: 8A61.0] | |||
| Molecule Alteration | SNP | rs12402969 C+ Genotypes CC+CT |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Genotyping assay | |||
| Mechanism Description | By analyzing the association between KCNJ10 polymorphisms and anti-epileptic drug efficacy of GGEs we found the frequency of rs12402969 C allele and CC+CT genotypes were higher in GGEs drug responsive patients than that in drug resistant patients | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
