Drug Information
Drug (ID: DG00638) and It's Reported Resistant Information
| Name |
Valproic acid
|
||||
|---|---|---|---|---|---|
| Synonyms |
VALPROIC ACID; 2-Propylpentanoic acid; 99-66-1; 2-Propylvaleric acid; Dipropylacetic acid; Depakine; Depakene; Valproate; Ergenyl; Di-n-propylacetic acid; Mylproin; Pentanoic acid, 2-propyl-; 4-Heptanecarboxylic acid; n-Dipropylacetic acid; Propylvaleric acid; Myproic Acid; n-DPA; Dipropylacetate; Convulex; Depakin; Di-n-propylessigsaure; Avugane; Baceca; Stavzor; Acido valproico; Kyselina 2-propylvalerova; Acide valproique; 2-n-Propyl-n-valeric acid; Acidum valproicum; Acetic acid, dipropyl-; 2,2-di-n-propylacetic acid; Valproinsaeure; Savicol; Depakine chrono; Depakin chrono; Abbott 44090; Acide valproique [INN-French]; Acido valproico [INN-Spanish]; Acidum valproicum [INN-Latin]; 2-PROPYL-PENTANOIC ACID; Di-n-propylessigsaure [German]; Valeric acid, 2-propyl-; Epilim; Kyselina 2-propylvalerova [Czech]; Di-n-propylessigsaeure; VPA; VPA;2-Propylpentanoic Acid; UNII-614OI1Z5WI; NSC 93819; Delepsine; Sprinkle; Valcote; Valparin; Epilex; PEAC; Depakene (TN); MFCD00002672; Vupral; CHEMBL109; (n-C3H7)2CHCOOH; 614OI1Z5WI; CHEBI:39867; Valerin; NSC-93819; NCGC00091149-01; Deproic; Alti-Valproic; Novo-Valproic; Penta-Valproic; Dom-Valproic; Med Valproic; Nu-Valproic; DSSTox_CID_3733; DSSTox_RID_77171; DSSTox_GSID_23733; Valproic acid USP; PMS-Valproic Acid; Valproic acid (USP); CAS-99-66-1; Depakote (TM); SMR000499581; HSDB 3582; 2 PP (base); EINECS 202-777-3; BRN 1750447; valproic-acid; Novo-divalproex; Sandoz valproic; Dom-valproate; Gen-divalproex; Apo-valproic; A-44090; AI3-10500; APO-divalproex; DOM-divalproex; Epival er; PHL-valproate; PMS-valproate; PMS-Divalproex; Erganyl; Stavzor; Dom-valproic acid; Apo-valproic syrup; PHL-valproic acid; Epiject I.V.; 2-propyl-Pentanoate; Epical (TM); Epilim (Salt/Mix); Valproic acid [USAN:USP:INN:BAN]; Depacon (Salt/Mix); Convulex (Salt/Mix); Eurekene (Salt/Mix); G2M-777; Valparin (Salt/Mix); Valproic acid solution; Novo-Valproic - ECC; Spectrum_000521; Divalproex (Salt/Mix); Dom-valproic acid syrup; Ratio-Valproic - ECC; Valdisoval (Salt/Mix); 2 -propylpentanoic acid; di-n-propyl acetic acid; S(-)-4-En-valproate; Spectrum2_000946; Spectrum3_001733; Spectrum4_000376; pentanoic acid, 2-propyl; DOM-valproic acid E.C.; PHL-valproic acid E.C.; PMS-valproic acid E.C.; Novo-valproic soft gel cap; EC 202-777-3; SCHEMBL2275; S-2-n-Propyl-4-pentenoate; (S)-2-propyl-4-pentanoate; KBioGR_000871; KBioGR_002277; KBioSS_001001; KBioSS_002278; MLS001076682; MLS001335927; MLS001335928; MLS002415770; BIDD:GT0858; DivK1c_000273; Valproic acid extended release; SPBio_000912; GTPL7009; DTXSID6023733; WLN: QVY3 & 3; KBio1_000273; KBio2_001001; KBio2_002277; KBio2_003569; KBio2_004845; KBio2_006137; KBio2_007413; KBio3_002626; KBio3_002757; NIJJYAXOARWZEE-UHFFFAOYSA-; Valproic acid [USAN:BAN:INN]; NINDS_000273; HMS2089J06; HMS2231E06; HMS3259C18; HMS3370C21; HMS3715B15; HMS3885G14; ACT05281; ALBB-032973; BCP33204; NSC93819; ZINC3008621; Tox21_111091; Tox21_201963; Tox21_300603; BDBM50003616; LMFA01020291; s3944; STL445581; AKOS009156895; Tox21_111091_1; Valproic Acid 1.0 mg/ml in Methanol; CCG-221127; CS-1765; DB00313; MCULE-7136317196; NC00584; SDCCGSBI-0050864.P004; NCGC00091149-02; NCGC00091149-03; NCGC00091149-04; NCGC00091149-05; NCGC00091149-06; NCGC00091149-08; NCGC00091149-09; NCGC00091149-26; NCGC00162288-07; NCGC00254365-01; NCGC00259512-01; AS-11354; HY-10585; SBI-0050864.P003; FT-0609289; FT-0675769; P0823; A19450; C07185; D00399; AB00698315-06; Q240642; Q-200321; Sodium valproate; 2-Propylpentanoic acid sodium salt; SR-01000075242-7; F2191-0115; Z1511532065; Valproic acid, European Pharmacopoeia (EP) Reference Standard; Valproic acid, United States Pharmacopeia (USP) Reference Standard; Valproic acid for system suitability, European Pharmacopoeia (EP) Reference Standard; Valproic acid solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Valproic acid, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
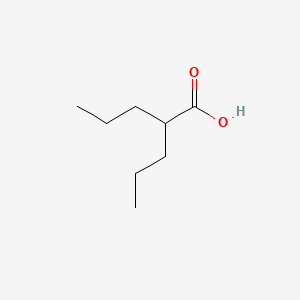
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[2]
|
||||
| Target | Glycogen synthase kinase-3 alpha (GSK-3A) | GSK3A_HUMAN | [1] | ||
| Histone deacetylase (HDAC) | NOUNIPROTAC | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C8H16O2
|
||||
| IsoSMILES |
CCCC(CCC)C(=O)O
|
||||
| InChI |
1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
|
||||
| InChIKey |
NIJJYAXOARWZEE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-08: Nervous system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Glutathione synthetase (GSH) | [2] | |||
| Resistant Disease | mitochondrial refractory epilepsy [ICD-11: 8A60.A] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vivo Model | Swiss albino mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
Ellman assay | |||
| Experiment for Drug Resistance |
Pre-treatment resistance testing; Post-treatment resistance testing | |||
| Mechanism Description | The involvement of complex I in drug resistance is well established in epilepsy; therefore, the model chosen for this study was rotenone corneal kindled model of drug resistance using rotenone as a selective irreversible inhibitor of complex I, which have shown resistance to drugs such as valproate, levetiracetam, lamotrigine, pregabalin, carbamazepine, zonisamide, topiramate, gabapentin and their combinations | |||
| Key Molecule: Quinone reductase 1 (NQO1) | [2] | |||
| Resistant Disease | mitochondrial refractory epilepsy [ICD-11: 8A60.A] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| Cell Pathway Regulation | Nrf2 signaling pathway | Inhibition | hsa05208 | |
| In Vivo Model | Swiss albino mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
ELISA assay | |||
| Experiment for Drug Resistance |
Pre-treatment resistance testing; Post-treatment resistance testing | |||
| Mechanism Description | The involvement of complex I in drug resistance is well established in epilepsy; therefore, the model chosen for this study was rotenone corneal kindled model of drug resistance using rotenone as a selective irreversible inhibitor of complex I, which have shown resistance to drugs such as valproate, levetiracetam, lamotrigine, pregabalin, carbamazepine, zonisamide, topiramate, gabapentin and their combinations | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Potassium inwardly rectifying channel subfamily J member 10 (KCNJ10) | [1] | |||
| Sensitive Disease | Genetic generalized epilepsies [ICD-11: 8A61.0] | |||
| Molecule Alteration | SNP | rs12402969 C+ Genotypes CC+CT |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Genotyping assay | |||
| Mechanism Description | By analyzing the association between KCNJ10 polymorphisms and anti-epileptic drug efficacy of GGEs we found the frequency of rs12402969 C allele and CC+CT genotypes were higher in GGEs drug responsive patients than that in drug resistant patients | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
