Drug Information
Drug (ID: DG00628) and It's Reported Resistant Information
| Name |
INK128
|
||||
|---|---|---|---|---|---|
| Synonyms |
1224844-38-5; Sapanisertib; INK-128; MLN0128; INK 128; INK128; TAK-228; INK 128 (MLN0128); Sapanisertib (MLN0128); MLN-0128; 5-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)benzo[d]oxazol-2-amine; UNII-JGH0DF1U03; 3-(2-Amino-5-benzoxazolyl)-1-(1-methylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; JGH0DF1U03; 5-(4-amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; 5-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]-pyrimidin-3-yl)benzo[d]oxazol-2-amine; C15H15N7O; 5-(4-azanyl-1-propan-2-yl-pyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; Sapanisertib; INK128; Sapanisertib (USAN/INN); Sapanisertib [USAN:INN]; MLS006011012; GTPL7933; SCHEMBL7902875; CHEMBL3545097; CHEBI:91450; EX-A951; INK-128/INK128; SYN1157; BDBM315477; HMS3656H12; HMS3672C21; INK-128;Sapanisertib;MLN0128; AOB87177; INK-0128; 2477AH; MFCD22124893; NSC764658; NSC768435; NSC780880; s2811; ZINC73069271; AKOS025149512; US10172858, Table 1.1; BCP9000789; CCG-265002; CS-0557; DB11836; NSC-764658; NSC-768435; NSC-780880; SB16566; US10172858, Table 1.22; NCGC00346654-01; NCGC00346654-10; AC-26848; AS-16294; BS170924; HY-13328; SMR004702810; BCP0726000086; A8551; FT-0700125; SW220210-1; D11183; INK-128,CAS:1224844-38-5; J-004811; Q27078072; 1H-Pyrazolo[3,4-d]pyrimidin-4-amine, 3-(2-amino-5-benzoxazolyl)-1-(1-methylethyl)-; 2-Benzoxazolamine, 5-(4-amino-1-(1-methylethyl)-1H-pyrazolo(3,4-d)pyrimidin-3-yl)-; 3-(2-Amino-1,3-benzoxazol-5-yl)-1-isopropyl-1H-pyrazolo(3,4-d)pyrimidin-4-amine; 5-(4-amino-1-isopropyl-pyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; FE5; INK-128; ; ; MLN-0128; ; ; 5-(4-Amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
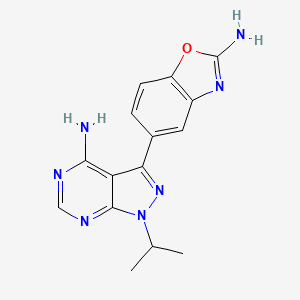
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Serine/threonine-protein kinase mTOR (mTOR) | MTOR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C15H15N7O
|
||||
| IsoSMILES |
CC(C)N1C2=NC=NC(=C2C(=N1)C3=CC4=C(C=C3)OC(=N4)N)N
|
||||
| InChI |
1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19)
|
||||
| InChIKey |
GYLDXIAOMVERTK-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Ubiquitin protein ligase E3 component n-recognin 5 (UBR5) | [1] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Breast cancer [ICD-11: 2C60] | |||
| The Specified Disease | Breast cancer | |||
| The Studied Tissue | Breast tissue | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 9.41E-80 Fold-change: 1.02E-01 Z-score: 2.18E+01 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | MCF-7 cells | Breast | Homo sapiens (Human) | CVCL_0031 |
| MDA-MB-436 cells | Breast | Homo sapiens (Human) | CVCL_0623 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTS assay | |||
| Mechanism Description | High nuclear EDD expression in a cohort of 151 women with serous ovarian carcinoma was associated with an increased risk of disease recurrence following first-line chemotherapy, and siRNA-knockdown of EDD gene expression partially restored cisplatin sensitivity in cisplatin-resistant ovarian cancer cells in vitro. Loss of EDD induced cell-cycle arrest at G1 through upregulation of tumour suppressor p53 and p21 proteins in osteosarcoma cells in vitro. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
