Drug Information
Drug (ID: DG00612) and It's Reported Resistant Information
| Name |
Hydrocortisone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Hydrocortisone; Cortisol; 50-23-7; Acticort; Cetacort; Hydrasson; Hydrocortisyl; Cobadex; Cortef; Hydrocortone; Hycort; Hytone; Signef; 17-Hydroxycorticosterone; Optef; Kendall's compound F; Cortanal; Cortenema; Corticreme; Cortifan; Cortiment; Cortispray; Cortonema; Dermacort; Dermolate; Efcorbin; Efcortelan; Ficortril; Genacort; Hycortol; Hycortole; Permicort; Proctocort; Tarcortin; Traumaide; Alacort; Cleiton; Cortril; Dihydrocostisone; Hytone lotion; Hidro-Colisona; Hydro-Adreson; Scheroson F; Incortin-H; Reichstein's substance M; Cort-Dome; Domolene-HC; Epiderm H; Esiderm H; Otosone-F; Polcort H; Cortolotion; Cortoxide; Cremesone; Eldercort; Heb-Cort; Maintasone; Delacort; Dioderm; Eldecort; Epicort; Mildison; Penecort; Rectoid; Anflam; Hydrocorticosterone; Hydroxycortisone; H-Cort; Hydro-Colisona; Cortisol alcohol; Incortin-hydrogen; Ala-Scalp; Aeroseb-HC; Barseb HC; Dermocortal; Nutracort; Synacort; Timocort; Evacort; Komed HC; Hydrocortisone base; Lacticare-HC; Texacort lotion 25; Ala-Cort; Anti-inflammatory hormone; Hydrocortisone alcohol; Algicirtis; Aquacort; Cortesal; Cortisolonum; Flexicort; Hidalone; Hytisone; Kyypakkaus; Lactisona; Lubricort; Meusicort; Milliderm; Sanatison; Schericur; Sigmacort; Stiefcorcil; Texacort; Amberin; Cutisol; Dermil; Uniderm; Foille Insetti; Gyno-Cortisone; Transderma H; Basan-Corti; Clear aid; Cremicort-H; Dome-cort; Neosporin-H Ear; Remederm HC; Aquanil HC; Cortisporin Otico; Derm-Aid; Heb Cort; Scalpicin Capilar; Systral Hydrocort; Prevex HC; Cortisporin; Efcortelin; Fiocortril; Hidrocortisona; Hydrocortisone free alcohol; Hydrocortisonum; Proctofoam; Alphaderm; Hydracort; Medicort; Otocort; Zenoxone; Drotic; Vytone; 11beta-Hydroxycortisone; Nystaform-HC; Aeroseb HC; CaldeCORT Spray; Pediotic Suspension; VoSol HC; Idrocortisone; 17alpha-Hydroxycorticosterone; Hydrocortal; Hydroskin; Otalgine; Otobiotic; Protocort; Hysone; Racet; Ef corlin; 11beta-Hydrocortisone; Compound F; Lacticare HC; Compound F (kendall); 11-beta-Hydrocortisone; 11-beta-Hydroxycortisone; Hydrocortisone (Cortisol); Chronocort; Hydrocort; Plenadren; Preparation H Hydrocortisone Cream; Neo-Cort-Dome; 11beta,17alpha,21-Trihydroxy-4-pregnene-3,20-dione; Otic-Neo-Cort-Dome; 11beta,17,21-Trihydroxypregn-4-ene-3,20-dione; NSC 10483; HC; UNII-WI4X0X7BPJ; [3H]cortisol; (11beta)-11,17,21-trihydroxypregn-4-ene-3,20-dione; Prestwick_265; (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one; 4-Pregnene-11beta,17alpha,21-triol-3,20-dione; CHEBI:17650; 11beta,17alpha,21-Trihydroxypregn-4-ene-3,20-dione; NSC10483; 11.beta.-Hydrocortisone; Dermaspray; MFCD00011654; NSC-10483; WI4X0X7BPJ; 11beta,17,21-Trihydroxyprogesterone; 11.beta.-Hydroxycortisone; Ophthocort; Terra-cortril; MLS000069609; 17.alpha.-Hydroxycorticosterone; Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11beta)-; 4-Pregnen-11beta,17alpha,21-triol-3,20-dione; Idrocortisone [DCIT]; Genacort (lotion); Anucort; Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11b)-; Prepcort; SMR000059022; DSSTox_CID_714; Hydrocortisonum [INN-Latin]; Proctozone HC; Scalp-Cort; Hidrocortisona [INN-Spanish]; Rectasol-HC; Anucort-HC; Hydro-RX; DSSTox_RID_75753; DSSTox_GSID_20714; (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; HC (HYDROCORTISONE); Corhydron; (11alpha,14beta)-11,17,21-Trihydroxypregn-4-Ene-3,20-Dione; DuoCort; HYDROCORTISONE IN ABSORBASE; Proctosol-HC; HC #1; HC #4; Acticort (TN); Colocort (TN); (1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one; SMR000653523; Cortef (TN); Hytone (TN); Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11.beta.)-; CCRIS 5854; component of Otalgine; Anusol HC (TN); component of Lubricort; COR-OTICIN; HSDB 3339; EINECS 200-020-1; component of Neo-Cort-Dome; Cortizol; Efmody; AI3-25006; 3h-cortisol; 11beta-cortisol; CAS-50-23-7; 11-Hydrocortisone; Plenadren (TN); NCGC00022848-06; 11b-Hydrocortisone; Kendalls compound F; Hydrocortisone [USP:INN:BAN:JAN]; Drotic (Salt/Mix); 11b-Hydroxycortisone; Otocort (Salt/Mix); Pregn-4-ene-3,20-dione, 11beta,17,21-trihydroxy-; Otalgine (Salt/Mix); Hydrocortisone, 98%; 11,17,21-Trihydroxypregn-4-ene-3,20-dione; Alphaderm (Salt/Mix); Hydrocortisone, topical; Otobiotic (Salt/Mix); Reichsteins substance M; 4p6x; Cort-Quin (Salt/Mix); Cortisporin (Salt/Mix); VoSol HC (Salt/Mix); 11a-Hydroxycorticosterone; 17a-Hydroxycorticosterone; Opera_ID_1292; Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11-beta)-; Prestwick0_000447; Prestwick1_000447; Prestwick2_000447; Prestwick3_000447; Epitope ID:174851; UPCMLD-DP133; EC 200-020-1; H 4001; SCHEMBL4148; Neo-Cort-Dome (Salt/Mix); Lopac0_000594; 11alpha-Hydroxycorticosterone; BSPBio_000494; MLS001148103; MLS002207135; MLS002222189; MLS002548868; SPBio_002433; BPBio1_000544; CHEMBL389621; GTPL2868; Pediotic Suspension (Salt/Mix); DTXSID7020714; UPCMLD-DP133:001; BDBM13775; Otic-Neo-Cort-Dome (Salt/Mix); 2v95; Hydrocortisone (JP17/USP/INN); HMS1569I16; HMS2090M04; HMS2096I16; HMS2230B18; HMS2235F17; HMS3259C05; HMS3261H10; HMS3713I16; Hydrocortisone, >=98% (HPLC); Vioform-Hydrocortisone (Salt/Mix); 11b,17,21-Trihydroxyprogesterone; BCP09054; HY-N0583; Tox21_110883; Tox21_200815; Tox21_500594; LMST02030001; s1696; ZINC13540519; AKOS001582651; Tox21_110883_1; CCG-204683; DB00741; LP00594; MCULE-8953627920; NC00456; SDCCGSBI-0050576.P003; 11.beta.,17,21-trihydroxyprogesterone; SMP1_000156; NCGC00022848-07; NCGC00022848-09; NCGC00022848-10; NCGC00022848-11; NCGC00022848-12; NCGC00022848-13; NCGC00022848-14; NCGC00022848-15; NCGC00022848-17; NCGC00022848-26; NCGC00258369-01; NCGC00261279-01; (1S,10S,11S,15S,17S,2R,14R)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyl tetracyclo[8.7.0.0<2,7>.0<11,15>]heptadec-6-en-5-one; (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one; AC-12902; AS-11651; BP-20390; NCI60_000118; 4-Pregnene-11alpha,21-triol 3,20-dione; B1951; EU-0100594; 4-Pregnene-11b,17a,21-triol-3,20-dione; 50H237; C00735; D00088; J10114; U 1851; Hydrocortisone, meets USP testing specifications; Pregn-4-ene-3, 11.beta.,17,21-trihydroxy-; 11 ,17 ,21-Trihydroxypregn-4-ene-3,20-dione; A929789; Hydrocortisone, VETRANAL(TM), analytical standard; Q190875; SR-01000000139; Q-201211; SR-01000000139-3; 11.beta.,17,21-Trihydroxypregn-4-ene-3,20-dione; BRD-K93568044-001-03-1; BRD-K93568044-001-11-4; BRD-K93568044-001-32-0; Hydrocortisone, BioReagent, suitable for cell culture; 4-Pregnen-11.beta.,17.alpha.,21-triol-3,20-dione; 4-Pregnene-11.beta.,17.alpha.,21-triol-3,20-dione; Pregn-4-ene-3,20-dione, 11.beta.,17,21-trihydroxy-; Z2786051549; (11beta)-11,17,21-Trihydroxy-pregn-4-ene-3,20-dione; 11.beta.,17.alpha.,21-Trihydroxy-4-pregnene-3,20-dione; 11.beta.,17.alpha.,21-Trihydroxypregn-4-ene-3,20-dione; B48448A1-24BA-47CA-8D9E-43E5BC949386; Hydrocortisone, British Pharmacopoeia (BP) Assay Standard; Pregn-4-ene-3, 11,17,21-trihydroxy-, (11.beta.)-; 11,17,21-Trihydroxypregn-4-ene-3,20-dione, (11.beta.)-; Hydrocortisone, European Pharmacopoeia (EP) Reference Standard; Hydrocortisone, United States Pharmacopeia (USP) Reference Standard; Hydrocortisone-Water Soluble, BioReagent, suitable for cell culture; Hydrocortisone, gamma-irradiated, powder, BioXtra, suitable for cell culture; 4-(6-chloro-4-oxoquinazolin-3(4H)-yl)-N-(3-methoxyphenyl)piperidine-1-carboxamide; Cortisol solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; Hydrocortisone for peak identification, European Pharmacopoeia (EP) Reference Standard; Hydrocortisone solution, 50 muM, sterile-filtered, BioXtra, suitable for cell culture; Hydrocortisone, Pharmaceutical Secondary Standard; Certified Reference Material; (10R,13S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
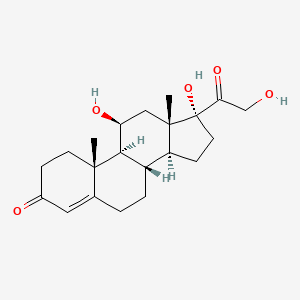
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(2 diseases)
[2]
[1]
|
||||
| Target | Glucocorticoid receptor (NR3C1) | GCR_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C21H30O5
|
||||
| IsoSMILES |
C[C@]12CCC(=O)C=C1CC[C@@H]3[C@@H]2[C@H](C[C@]4([C@H]3CC[C@@]4(C(=O)CO)O)C)O
|
||||
| InChI |
1S/C21H30O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h9,14-16,18,22,24,26H,3-8,10-11H2,1-2H3/t14-,15-,16-,18+,19-,20-,21-/m0/s1
|
||||
| InChIKey |
JYGXADMDTFJGBT-VWUMJDOOSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: CAMPATH-1 antigen (CD52) | [3] | |||
| Sensitive Disease | t-cell prolymphocytic leukemia [ICD-11: 2A90.0] | |||
| Molecule Alteration | Expressiom | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | T-cell prolymphocytic leukemia patient | Homo sapiens | ||
| Experiment for Molecule Alteration |
Flow cytometry | |||
| Experiment for Drug Resistance |
Overall survival assay | |||
| Mechanism Description | MTX-HOPE is a combination of classical chemotherapy agents originally developed for palliative chemotherapy in frail patients with refractory lymphoma. MTX-HOPE has been reported to be effective against T-cell tumors. Severe nonhematologic adverse events are rarely reported; however, bone marrow suppression is commonly observed. | |||
ICD-12: Respiratory system diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [2] | |||
| Resistant Disease | Chronic inflammatory lung disease [ICD-11: CA40.Z] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | For therapeutic drugs to be effective at reducing the proinflammatory/cytotoxic potential of steroid resistant lymphocytes, glucocorticoids enter cells by overcoming membrane drug efflux pump P-glycoprotein-1 (Pgp1) and binding to the glucocorticoid receptor (GCR) in the cytoplasm. GCR must be bound to the molecular chaperones heat shock proteins (Hsp)70 and Hsp90 to acquire a high-affinity steroid binding conformation, and trafficked to the nucleus where engagement of histone deacetylases (HDACs), particularly HDAC2, results in the reduction of pro-inflammatory gene activation. | |||
ICD-15: Musculoskeletal/connective-tissue diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Multidrug resistance protein 1 (ABCB1) | [1] | |||
| Resistant Disease | Rheumatoid arthritis [ICD-11: FA20.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Mechanism Description | MTX is a substrate for eight ABC transporters. In vitro studies demonstrated that RAFLS treated with MTX had higher ABCB1 expression levels than controls, with a positive correlation between ABCB1 expression levels and RA treatment duration. In addition to MTX, other DMARDs (e.g. sulfasalazine, leflunomide, bucillamine, azathioprine), glucocorticoids (e.g. betamethasone, dexamethasone), and NSAIDs (e.g. celecoxib and indomethacin) are also substrates of ABC transporters. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
