Drug Information
Drug (ID: DG00570) and It's Reported Resistant Information
| Name |
Flomoxef
|
||||
|---|---|---|---|---|---|
| Synonyms |
Flomoxef; 99665-00-6; Flomoxefum; FMOX; Flomoxef [INN]; UNII-V9E5U5XF42; V9E5U5XF42; Flomoxef (INN); flomoxef sodium; (6R,7R)-7-[[2-(difluoromethylsulfanyl)acetyl]amino]-3-[[1-(2-hydroxyethyl)tetrazol-5-yl]sulfanylmethyl]-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; Flomoxefo; 5-Oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[(difluoromethyl)thio]acetyl]amino]-3-[[[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]thio]methyl]-7-methoxy-8-oxo-, (6r-cis)-; 5-Oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[[(difluoromethyl)thio]acetyl]amino]-3-[[[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]thio]methyl]-7-methoxy-8-oxo-, (6R,7R)-; Flomoxefum [Latin]; Flomoxefo [Spanish]; C15H18F2N6O7S2; NCGC00182999-01; SCHEMBL49438; CHEMBL15413; CHEBI:135813; BCP20920; HY-B0706; ZINC3874302; AKOS015896442; DB11935; AC-15832; K789; CS-0009596; D07963; 665F006; A846063; Q5459999; (-)-(6R,7R)-7-(2-((Difluoromethyl)thio)acetamido)-3-(((1-(2-hydroxyethyl)-1H-tetrazol-5-yl)thio)methyl)-7-methoxy-8-oxo-5-oxa-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[2-(difluoromethylthio)-1-oxoethyl]amino]-3-[[[1-(2-hydroxyethyl)-5-tetrazolyl]thio]methyl]-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[2-[bis(fluoranyl)methylsulfanyl]ethanoylamino]-3-[[1-(2-hydroxyethyl)-1,2,3,4-tetrazol-5-yl]sulfanylmethyl]-7-methoxy-8-oxidanylidene-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-{2-[(difluoromethyl)sulfanyl]acetamido}-3-({[1-(2-hydroxyethyl)-1H-tetrazol-5-yl]sulfanyl}methyl)-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (7R)-7-(2-(difluoromethylthio)acetamido)-3-((1-(2-hydroxyethyl)-1H-tetrazol-5-ylthio)methyl)-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
Click to Show/Hide
|
||||
| Structure |
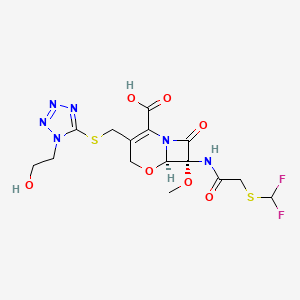
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C15H18F2N6O7S2
|
||||
| IsoSMILES |
CO[C@@]1([C@@H]2N(C1=O)C(=C(CO2)CSC3=NN=NN3CCO)C(=O)O)NC(=O)CSC(F)F
|
||||
| InChI |
1S/C15H18F2N6O7S2/c1-29-15(18-8(25)6-31-13(16)17)11(28)23-9(10(26)27)7(4-30-12(15)23)5-32-14-19-20-21-22(14)2-3-24/h12-13,24H,2-6H2,1H3,(H,18,25)(H,26,27)/t12-,15+/m1/s1
|
||||
| InChIKey |
UHRBTBZOWWGKMK-DOMZBBRYSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: OXA-23 carbapenemase (BLA OXA-23) | [1] | |||
| Resistant Disease | Cutaneous bacterial infection [ICD-11: 1B21.4] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Acinetobacter baumannii isolates | 470 | ||
| Experiment for Molecule Alteration |
qRT-PCR | |||
| Experiment for Drug Resistance |
Broth microdilution method assay; Agar dilution method assay | |||
| Mechanism Description | The isolate was resistant to antibiotics other than ampicillin-sulbactam and colistin, suggesting drug resistance due to carbapenemase production by OXA-23.carbapenem resistance in the isolated carbapenem-resistant A. baumannii strain was at least partially conferred by bla OXA-23-like carbapenemase. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
