Drug Information
Drug (ID: DG00438) and It's Reported Resistant Information
| Name |
Toremifene
|
||||
|---|---|---|---|---|---|
| Synonyms |
Acapodene; Estrimex; Farestone; Toremifeno; Toremifenum; Toremifene Base; Toremifeno [Spanish]; Toremifenum [Latin]; GTx 006; Acapodene (TN); FC-1157a; Fareston (TN); GTx-006; Toremifene (INN); Toremifene [INN:BAN]; Z-Toremifene; GTX-006 (Acapodene); Toremifene Citrate (1:1); {2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethyl}-dimethyl-amine; (Z)-2-(4-(4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine; 2-(p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]-phenoxy)-N,N-dimethyl-ethylamine citrate(1:1); 2-(para-((Z)-4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethylamine (IUPAC); 2-({4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenyl}oxy)-N,N-dimethylethanamine; 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine; 2-{4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
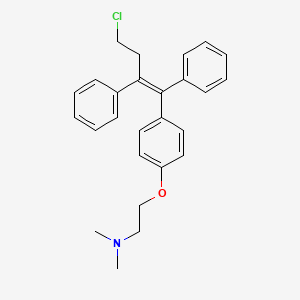
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Estrogen receptor (ESR) | ESR1_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C26H28ClNO
|
||||
| IsoSMILES |
CN(C)CCOC1=CC=C(C=C1)/C(=C(/CCCl)\\C2=CC=CC=C2)/C3=CC=CC=C3
|
||||
| InChI |
1S/C26H28ClNO/c1-28(2)19-20-29-24-15-13-23(14-16-24)26(22-11-7-4-8-12-22)25(17-18-27)21-9-5-3-6-10-21/h3-16H,17-20H2,1-2H3/b26-25-
|
||||
| InChIKey |
XFCLJVABOIYOMF-QPLCGJKRSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Estrogen receptor alpha (ESR1) | [1] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Missense mutation | p.Y537C |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | ||
| Experiment for Molecule Alteration |
Droplet digital polymerase chain reaction assay | |||
| Experiment for Drug Resistance |
Chest x-ray assay; Computed tomography assay; Magnetic resonance imaging assay; Positron emission tomography assay | |||
| Mechanism Description | We have developed a ddPCR-based method for the sensitive detection and quantification of 4 representative ESR1 mutations, Y537S, Y537N, Y537C, and D538G, in 325 breast cancer specimens, in which 270 primary breast cancer and 55 MBC specimens. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
