Drug Information
Drug (ID: DG00426) and It's Reported Resistant Information
| Name |
Enrofloxacin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Enrofloxacin; 93106-60-6; Baytril; Enrofloxacine; CFPQ; Enrofloxacino; Enrofloxacinum; BAY VP 2674; 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; Enroxil; UNII-3DX3XEK1BN; Baytril (TN); N-Ethylciprofloxacin; ERFX; 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; C19H22FN3O3; MFCD00792463; 3DX3XEK1BN; 3-Quinolinecarboxylic acid, 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-; MLS000069441; CHEBI:35720; NSC-758616; BAY Vp 2674;PD160788; endrofloxicin; NCGC00018143-04; CPD000059011; PD160788; SMR000059011; 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxoquinoline-3-carboxylic acid; DSSTox_CID_25619; DSSTox_RID_81007; DSSTox_GSID_45619; 3-Quinolinecarboxylic acid, 1,4-dihydro-1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-; Enrofloxacine [French]; Enrofloxacinum [Latin]; Enrofloxacino [Spanish]; Bay-Vp-2674; 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic Acid; CAS-93106-60-6; Enrofloxacin [USAN:BAN:INN]; HSDB 6952; BRN 5307824; CCRIS 8214; Enrofloxacin [USAN:USP:INN:BAN]; Enrofloxacin-[d5]; Opera_ID_1106; Enrofloxacin (USP/INN); Enrofloxacin (USAN/INN); 1,4-Dihydro-1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-3-quinolinecarboxylic acid; BAY-Vp2674; MLS001076496; MLS001424169; CHEMBL15511; SCHEMBL149150; SPECTRUM1503721; Enrofloxacin for veterinary use; DTXSID1045619; HMS2052O09; HMS2090E12; HMS2093I21; HMS2234M11; HMS3373P14; HMS3394O09; HMS3715B18; Pharmakon1600-01503721; ZINC597112; ALBB-030792; BCP15457; HY-B0502; Enrofloxacin, >=98.0% (HPLC); Tox21_110831; BBL009982; DL-384; HTS028366; NSC758616; s3059; STK711109; AKOS005530685; BAY-Vp2674;PD160788; Tox21_110831_1; AC-7614; CCG-101102; DB11404; KS-5010; MCULE-6446074322; NC00352; NSC 758616; 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoro-4-oxo-quinoline-3-carboxylic acid; 1-cyclopropyl-7-(4-ethylpiperazinyl)-6-fluoro-4-oxohydroquinoline-3-carboxylic acid; NCGC00018143-01; NCGC00018143-02; NCGC00018143-03; NCGC00018143-05; NCGC00021632-03; H734; SBI-0206725.P001; AB0013220; DB-057368; B1742; E0786; FT-0625663; FT-0667862; D02473; AB00384269-16; AB00384269_17; AB00384269_18; Enrofloxacin, VETRANAL(TM), analytical standard; 106E606; A844445; Q414413; SR-01000000045; SR-01000000045-3; BRD-K76534306-001-11-0; Enrofloxacin, European Pharmacopoeia (EP) Reference Standard; Enrofloxacin, United States Pharmacopeia (USP) Reference Standard; 1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-4-oxo-3-quinolinecarboxylic acid; Enrofloxacin for system suitability, European Pharmacopoeia (EP) Reference Standard; Enrofloxacin, Pharmaceutical Secondary Standard; Certified Reference Material; 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinoline-carboxylic acid; 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-ethyl-1-piperazinyl)-3-quinolinecarboxylic acid; 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3- quinolonecarboxylic acid; 1-Cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid, 9CI; 1-cyclopropyl-7-(4-ethylpiperazin-1-yl)-6-fluoranyl-4-oxidanylidene-quinoline-3-carboxylic acid
Click to Show/Hide
|
||||
| Structure |
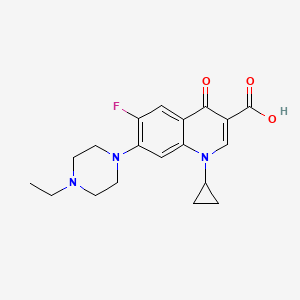
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C19H22FN3O3
|
||||
| IsoSMILES |
CCN1CCN(CC1)C2=C(C=C3C(=C2)N(C=C(C3=O)C(=O)O)C4CC4)F
|
||||
| InChI |
1S/C19H22FN3O3/c1-2-21-5-7-22(8-6-21)17-10-16-13(9-15(17)20)18(24)14(19(25)26)11-23(16)12-3-4-12/h9-12H,2-8H2,1H3,(H,25,26)
|
||||
| InChIKey |
SPFYMRJSYKOXGV-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Outer membrane porin (OMP38) | [1], [2] | |||
| Resistant Disease | Melioidosis [ICD-11: 1C42.0] | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Escherichia coli | 668369 | ||
| Escherichia coli BL21(DE3) | 469008 | |||
| Burkholderia pseudomallei isolates | 28450 | |||
| Experiment for Molecule Alteration |
Whole genome sequence assay | |||
| Experiment for Drug Resistance |
Broth microdilution method assay | |||
| Mechanism Description | Bps is highly resistant to many antimicrobial agents and this resistance may result from the low drug permeability of outer membrane proteins, known as porins.An Escherichia coli strain defective in most porins, but expressing BpsOmp38, exhibited considerably lower antimicrobial susceptibility than the control strain. In addition, mutation of Tyr119, the most prominent pore-lining residue in BpsOmp38, markedly altered membrane permeability, substitution with Ala (mutant BpsOmp38Y119A) enhanced uptake of the antimicrobial agents, while substitution with Phe (mutant BpsOmp38Y119F) inhibited uptake. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
