Drug Information
Drug (ID: DG00422) and It's Reported Resistant Information
| Name |
Exemestane
|
||||
|---|---|---|---|---|---|
| Synonyms |
Aromasil; Aromasin; Aromasine; EXE; Exemestance; Exemestano; Exemestanum; Nikidess; Pfizer brand of exemestane; Curator_000009; Fce 24304; Aromasin (TN); Aromasin, Exemestane; Exemestano [INN-Spanish]; Exemestanum [INN-Latin]; FCE-24304; PNU-155971; Exemestane [USAN:INN:BAN]; Exemestane (JAN/USP/INN); (8R,9S,10R,13S,14S)-10,13-dimethyl-6-methylidene-7,8,9,11,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,17-dione; 6-Methylenandrosta-1,4-diene-3,17-dione; 6-Methyleneandrosta-1,4-diene-3,17-dione; 6-methylideneandrosta-1,4-diene-3,17-dione
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
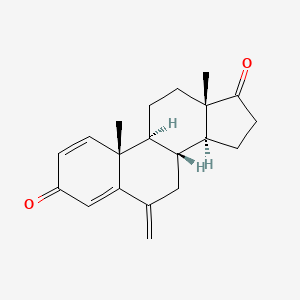
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
|
||||
| Target | Aromatase (CYP19A1) | CP19A_HUMAN | [2] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C20H24O2
|
||||
| IsoSMILES |
C[C@]12CC[C@H]3[C@H]([C@@H]1CCC2=O)CC(=C)C4=CC(=O)C=C[C@]34C
|
||||
| InChI |
1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1
|
||||
| InChIKey |
BFYIZQONLCFLEV-DAELLWKTSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Estrogen receptor alpha (ESR1) | [1], [2] | ||||||||||||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y537S |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.60 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.50 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
M
-
D
-
P
-
M
-
I
-
K
300
|
-
R
-
S
-
K
-
K
-
N
-
S
-
L
-
A
-
L
S
S
310
|
L
L
T
T
A
A
D
D
Q
Q
M
M
V
V
S
S
A
A
L
L
320
|
L
L
D
D
A
A
E
E
P
P
P
P
I
I
L
L
Y
Y
S
S
330
|
E
E
Y
Y
D
D
P
P
T
T
R
R
P
P
F
F
S
S
E
E
340
|
A
A
S
S
M
M
M
M
G
G
L
L
L
L
T
T
N
N
L
L
350
|
A
A
D
D
R
R
E
E
L
L
V
V
H
H
M
M
I
I
N
N
360
|
W
W
A
A
K
K
R
R
V
V
P
P
G
G
F
F
V
V
D
D
370
|
L
L
T
T
L
L
H
H
D
D
Q
Q
V
V
H
H
L
L
L
L
380
|
E
E
C
-
A
A
W
W
L
L
E
E
I
I
L
L
M
M
I
I
390
|
G
G
L
L
V
V
W
W
R
R
S
S
M
M
E
E
H
H
P
P
400
|
G
G
K
K
L
L
L
L
F
F
A
A
P
P
N
N
L
L
L
L
410
|
L
L
D
D
R
R
N
N
Q
Q
G
G
K
K
C
-
V
V
E
E
420
|
G
G
M
M
V
V
E
E
I
I
F
F
D
D
M
M
L
L
L
L
430
|
A
A
T
T
S
S
S
S
R
R
F
F
R
R
M
M
M
M
N
N
440
|
L
L
Q
Q
G
G
E
E
E
E
F
F
V
V
C
C
L
L
K
K
450
|
S
S
I
I
I
I
L
L
L
L
N
N
S
S
G
G
V
V
Y
Y
460
|
T
T
F
F
L
L
S
S
S
S
T
T
L
L
K
K
S
S
L
L
470
|
E
E
E
E
K
K
D
D
H
H
I
I
H
H
R
R
V
V
L
L
480
|
D
D
K
K
I
I
T
T
D
D
T
T
L
L
I
I
H
H
L
L
490
|
M
M
A
A
K
K
A
A
G
G
L
L
T
T
L
L
Q
Q
Q
Q
500
|
Q
Q
H
H
Q
Q
R
R
L
L
A
A
Q
Q
L
L
L
L
L
L
510
|
I
I
L
L
S
S
H
H
I
I
R
R
H
H
M
M
S
S
N
N
520
|
K
K
G
G
M
M
E
E
H
H
L
L
Y
Y
S
S
M
M
K
K
530
|
C
-
K
K
N
N
V
V
V
V
P
P
L
L
Y
S
D
D
L
L
540
|
L
L
L
L
E
E
M
M
L
L
D
D
A
A
H
H
R
R
L
L
550
|
H
H
A
A
P
P
T
T
S
S
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Droplet digital polymerase chain reaction assay | ||||||||||||
| Experiment for Drug Resistance |
Chest x-ray assay; Computed tomography assay; Magnetic resonance imaging assay; Positron emission tomography assay | ||||||||||||
| Mechanism Description | We have developed a ddPCR-based method for the sensitive detection and quantification of 4 representative ESR1 mutations, Y537S, Y537N, Y537C, and D538G, in 325 breast cancer specimens, in which 270 primary breast cancer and 55 MBC specimens. | ||||||||||||
| Key Molecule: Estrogen receptor alpha (ESR1) | [2], [3] | ||||||||||||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | ||||||||||||
| Molecule Alteration | Missense mutation | p.D538G |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 1.60 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 1.90 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
-
V
-
D
-
L
-
G
-
T
-
E
290
|
-
N
-
L
-
Y
-
F
-
Q
-
S
-
N
-
A
-
M
-
K
300
|
-
R
-
S
-
K
-
K
-
N
-
S
-
L
-
A
-
L
S
S
310
|
L
L
T
T
A
A
D
D
Q
Q
M
M
V
V
S
S
A
A
L
L
320
|
L
L
D
D
A
A
E
E
P
P
P
P
I
I
L
L
Y
Y
S
S
330
|
E
E
Y
Y
D
D
P
P
T
T
R
R
P
P
F
F
S
S
E
E
340
|
A
A
S
S
M
M
M
M
G
G
L
L
L
L
T
T
N
N
L
L
350
|
A
A
D
D
R
R
E
E
L
L
V
V
H
H
M
M
I
I
N
N
360
|
W
W
A
A
K
K
R
R
V
V
P
P
G
G
F
F
V
V
D
D
370
|
L
L
T
T
L
L
H
H
D
D
Q
Q
V
V
H
H
L
L
L
L
380
|
E
E
C
C
A
A
W
W
L
L
E
E
I
I
L
L
M
M
I
I
390
|
G
G
L
L
V
V
W
W
R
R
S
S
M
M
E
E
H
H
P
P
400
|
G
G
K
K
L
L
L
L
F
F
A
A
P
P
N
N
L
L
L
L
410
|
L
L
D
D
R
R
N
N
Q
Q
G
G
K
K
C
C
V
V
E
E
420
|
G
G
M
M
V
V
E
E
I
I
F
F
D
D
M
M
L
L
L
L
430
|
A
A
T
T
S
S
S
S
R
R
F
F
R
R
M
M
M
M
N
N
440
|
L
L
Q
Q
G
G
E
E
E
E
F
F
V
V
C
C
L
L
K
K
450
|
S
S
I
I
I
I
L
L
L
L
N
N
S
S
G
G
V
V
Y
Y
460
|
T
T
F
F
L
L
S
S
S
S
T
T
L
L
K
K
S
S
L
L
470
|
E
E
E
E
K
K
D
D
H
H
I
I
H
H
R
R
V
V
L
L
480
|
D
D
K
K
I
I
T
T
D
D
T
T
L
L
I
I
H
H
L
L
490
|
M
M
A
A
K
K
A
A
G
G
L
L
T
T
L
L
Q
Q
Q
Q
500
|
Q
Q
H
H
Q
Q
R
R
L
L
A
A
Q
Q
L
L
L
L
L
L
510
|
I
I
L
L
S
S
H
H
I
I
R
R
H
H
M
M
S
S
N
N
520
|
K
K
G
G
M
M
E
E
H
H
L
L
Y
Y
S
S
M
M
K
K
530
|
C
C
K
K
N
N
V
V
V
V
P
P
L
L
Y
Y
D
G
L
L
540
|
L
L
L
L
E
E
M
M
L
L
D
D
A
A
H
H
R
R
L
L
550
|
H
H
A
A
P
P
T
T
S
S
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Droplet digital polymerase chain reaction assay | ||||||||||||
| Experiment for Drug Resistance |
Chest x-ray assay; Computed tomography assay; Magnetic resonance imaging assay; Positron emission tomography assay | ||||||||||||
| Mechanism Description | We have developed a ddPCR-based method for the sensitive detection and quantification of 4 representative ESR1 mutations, Y537S, Y537N, Y537C, and D538G, in 325 breast cancer specimens, in which 270 primary breast cancer and 55 MBC specimens. | ||||||||||||
| Key Molecule: Estrogen receptor alpha (ESR1) | [2] | ||||||||||||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y537N |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Droplet digital polymerase chain reaction assay | ||||||||||||
| Experiment for Drug Resistance |
Chest x-ray assay; Computed tomography assay; Magnetic resonance imaging assay; Positron emission tomography assay | ||||||||||||
| Mechanism Description | We have developed a ddPCR-based method for the sensitive detection and quantification of 4 representative ESR1 mutations, Y537S, Y537N, Y537C, and D538G, in 325 breast cancer specimens, in which 270 primary breast cancer and 55 MBC specimens. | ||||||||||||
| Key Molecule: Estrogen receptor alpha (ESR1) | [2] | ||||||||||||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | ||||||||||||
| Molecule Alteration | Missense mutation | p.Y537C |
|||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| In Vivo Model | A retrospective survey in conducting clinical studies | Homo sapiens | |||||||||||
| Experiment for Molecule Alteration |
Droplet digital polymerase chain reaction assay | ||||||||||||
| Experiment for Drug Resistance |
Chest x-ray assay; Computed tomography assay; Magnetic resonance imaging assay; Positron emission tomography assay | ||||||||||||
| Mechanism Description | We have developed a ddPCR-based method for the sensitive detection and quantification of 4 representative ESR1 mutations, Y537S, Y537N, Y537C, and D538G, in 325 breast cancer specimens, in which 270 primary breast cancer and 55 MBC specimens. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
