Drug Information
Drug (ID: DG00016) and It's Reported Resistant Information
| Name |
Cephapirin
|
||||
|---|---|---|---|---|---|
| Synonyms |
CEPR; Cefa; Cefadyl; Cefapirin; Cefapirina; Cefapirine; Cefapirinum; Cefaprin; Cephapirine; Metricure; CEPHAPIRIN SODIUM; Cefaprinsodium; Cephapirin Monosodium Salt; ANTIBIOTIC BL-P1322; BL-P 1322; Cefa-ak; Cefadyl (TN); Cefapirin (BAN); Cefapirin [INN:BAN]; Cefapirina [INN-Spanish]; Cefapirine [INN-French]; Cefapirinum [INN-Latin]; Metricure (TN); (6R,7R)-3-(Acetoxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure; (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-pyridin-4-ylsulfanylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(acetyloxy)methyl]-8-oxo-7-{[(pyridin-4-ylthio)acetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-acetoxymethyl-7-[(pyridin-4-ylsulfanyl)acetamido]-3,4-didehydrocepham-4-carboxylic acid; 3-(Hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamidol-5-thia-1-azabicyclo(4.2.0)oct-2-en-2carbonsaeure acetat; 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-(hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-, acetate (ester); 7-(2-(4-Pyridylthio)acetamido)cephalosporanic acid
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
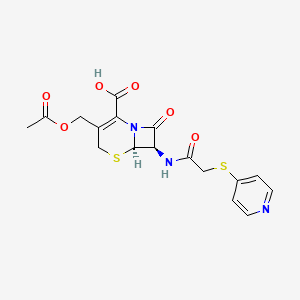
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Resistance Information Validated by in-vivo Model for This Drug
(1 diseases)
[1]
|
||||
| Target | Bacterial Penicillin binding protein (Bact PBP) | NOUNIPROTAC | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C17H17N3O6S2
|
||||
| IsoSMILES |
CC(=O)OCC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)CSC3=CC=NC=C3)SC1)C(=O)O
|
||||
| InChI |
1S/C17H17N3O6S2/c1-9(21)26-6-10-7-28-16-13(15(23)20(16)14(10)17(24)25)19-12(22)8-27-11-2-4-18-5-3-11/h2-5,13,16H,6-8H2,1H3,(H,19,22)(H,24,25)/t13-,16-/m1/s1
|
||||
| InChIKey |
UQLLWWBDSUHNEB-CZUORRHYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-01: Infectious/parasitic diseases
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Beta-lactamase (BLA) | [1] | |||
| Resistant Disease | Rhodobacter sphaeroides infection [ICD-11: 1A00-1C4Z] | |||
| Molecule Alteration | Expression | Inherence |
||
| Experimental Note | Discovered Using In-vivo Testing Model | |||
| In Vitro Model | Rhodopseudomonas sphaeroides strain DSM 160(Y) | 1063 | ||
| Rhodopseudomonas sphaeroides strain DSM158 | 1063 | |||
| Rhodopseudomonas sphaeroides strain DSM159 | 1063 | |||
| Experiment for Molecule Alteration |
Sodium dodecyl sulfate-PAGE assay | |||
| Experiment for Drug Resistance |
MIC assay | |||
| Mechanism Description | Thirteen strains of the gram-negative, facultative phototrophic bacterium Rhodobacter sphaeroides were examined fro susceptibility to beta-lactam antibiotics. All strains were sensitive to the semisynthetic penicillins ampicillin, carbenicillin, oxacillin, cloxacillin, and methicillin, but 10 of the 13 strains were resistant to penicillin G, as well as a number of cephalosporins, such as cephalothin, cephapirin, and cephalosporin C. A beta-lactamase (EC 3.5.2.6) with strong cephalosporinase activity was detected in all of the resistant strains of R. sphaeroides. With strain Y-1 as a model, it was shown that the beta-lactamase was inducible by penicillin G, cephalosporin C, cephalothin, and to some minor extent, cephapirin. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
