Drug Information
Drug (ID: DG00003) and It's Reported Resistant Information
| Name |
Capecitabine
|
||||
|---|---|---|---|---|---|
| Synonyms |
Capecitabin; Capecitabina; Capecitabinum; Capecitibine; Capiibine; Caxeta; Xabine; Xeloda; Capecitabine [USAN]; R340;R-340; RG-340; Ro 09-1978; Xeloda (TN); Ro 09-1978/000; Ro-09-1978; Xeloda, Captabin, Capecitabine; Capecitabine (JAN/USAN/INN); Ro-09-1978/000; N(4)-Pentyloxycarbonyl-5'-deoxy-5-fluorocytidine; Pentyl 1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate; Pentyl [1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]carbamate; Carbamic acid, (1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-, pentyl ester; Pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate; (1-(5-Deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-carbamic acid pentyl ester; 5'-Deoxy-5-fluoro-N-((pentyloxy)carbonyl)cytidine; 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine; Capecitabine (Fluoropyrimidine)
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
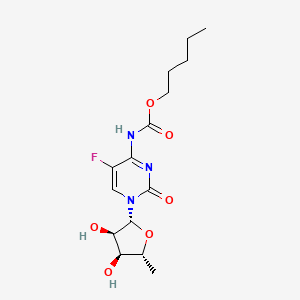
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(2 diseases)
[2]
[1]
|
||||
| Target | Candida Thymidylate synthase (Candi TMP1) | TYSY_CANAL | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
C15H22FN3O6
|
||||
| IsoSMILES |
CCCCCOC(=O)NC1=NC(=O)N(C=C1F)[C@H]2[C@@H]([C@@H]([C@H](O2)C)O)O
|
||||
| InChI |
1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
|
||||
| InChIKey |
GAGWJHPBXLXJQN-UORFTKCHSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| INTEDE ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: hsa-mir-296 | [1] | |||
| Resistant Disease | Colon cancer [ICD-11: 2B90.1] | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
qPCR | |||
| Experiment for Drug Resistance |
Response evaluation criteria in solid tumors assay | |||
| Mechanism Description | The patients with decrease in miR-296 at 4 weeks may reflect a more aggressive tumor phenotype with increased metastasis and tumor cell invasiveness. The loss of miR-296 may be one of the mechanisms for primary resistance of colorectal cancer to chemotherapy. | |||
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Growth arrest-specific protein 6 (GAS6) | [2] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Splicing mutation | Splicing |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AXLK signaling pathway | Activation | hsa01521 | |
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Circulating-free DNA assay; Whole exome sequencing assay | |||
| Mechanism Description | Quantification of allele fractions in plasma identified increased representation of mutant alleles in association with emergence of therapy resistance. | |||
| Key Molecule: Tumor protein 63 (TP63) | [2] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Missense mutation | p.S551G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | AXLK signaling pathway | Activation | hsa01521 | |
| In Vitro Model | Plasma | Blood | Homo sapiens (Human) | N.A. |
| Experiment for Molecule Alteration |
Circulating-free DNA assay; Whole exome sequencing assay | |||
| Mechanism Description | Quantification of allele fractions in plasma identified increased representation of mutant alleles in association with emergence of therapy resistance. | |||
| Key Molecule: Tumor protein 63 (TP63) | [2] | |||
| Resistant Disease | Breast cancer [ICD-11: 2C60.3] | |||
| Molecule Alteration | Missense mutation | p.S551G |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Experiment for Molecule Alteration |
Circulating-free DNA assay; Whole exome sequencing assay | |||
| Mechanism Description | Quantification of allele fractions in plasma identified increased representation of mutant alleles in association with emergence of therapy resistance. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
