Molecule Information
General Information of the Molecule (ID: Mol01960)
| Name |
Nuclear protein 1, transcriptional regulator (NUPR1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
NUPR1; COM1
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
NUPR1
|
||||
| Gene ID | |||||
| Location |
chr16:28,532,708-28,539,008[-]
|
||||
| Sequence |
MATFPPATSAPQQPPGPEDEDSSLDESDLYSLAHSYLGGGGRKGRTKREAAANTNRPSPG
GHERKLVTKLQNSERKKRGARR Click to Show/Hide
|
||||
| Function |
Transcription regulator that converts stress signals into a program of gene expression that empowers cells with resistance to the stress induced by a change in their microenvironment. Thereby participates in regulation of many process namely cell-cycle, apoptosis, autophagy and DNA repair responses. Controls cell cycle progression and protects cells from genotoxic stress induced by doxorubicin through the complex formation with TP53 and EP300 that binds CDKN1A promoter leading to transcriptional induction of CDKN1A. Protects pancreatic cancer cells from stress-induced cell death by binding the RELB promoter and activating its transcription, leading to IER3 transactivation. Negatively regulates apoptosis through interaction with PTMA. Inhibits autophagy-induced apoptosis in cardiac cells through FOXO3 interaction, inducing cytoplasmic translocation of FOXO3 thereby preventing the FOXO3 association with the pro-autophagic BNIP3 promoter. Inhibits cell growth and facilitates programmed cell death by apoptosis after adriamycin-induced DNA damage through transactivation of TP53. Regulates methamphetamine-induced apoptosis and autophagy through DDIT3-mediated endoplasmic reticulum stress pathway. Participates in DNA repair following gamma-irradiation by facilitating DNA access of the transcription machinery through interaction with MSL1 leading to inhibition of histone H4' Lys-16' acetylation (H4K16ac). Coactivator of PAX2 transcription factor activity, both by recruiting EP300 to increase PAX2 transcription factor activity and by binding PAXIP1 to suppress PAXIP1-induced inhibition on PAX2. Positively regulates cell cycle progression through interaction with COPS5 inducing cytoplasmic translocation of CDKN1B leading to the CDKN1B degradation. Coordinates, through its interaction with EP300, the assiociation of MYOD1, EP300 and DDX5 to the MYOG promoter, leading to inhibition of cell-cycle progression and myogenic differentiation promotion. Negatively regulates beta cell proliferation via inhibition of cell-cycle regulatory genes expression through the suppression of their promoter activities. Also required for LHB expression and ovarian maturation. Exacerbates CNS inflammation and demyelination upon cuprizone treatment.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
1 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Multiple myeloma [ICD-11: 2A83.0] | [1] | |||
| Sensitive Disease | Multiple myeloma [ICD-11: 2A83.0] | |||
| Sensitive Drug | Trifluoperazine | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Multiple myeloma [ICD-11: 2A83] | |||
| The Specified Disease | Myeloma | |||
| The Studied Tissue | Bone marrow | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.83E-03 Fold-change: -1.04E-01 Z-score: -4.20E+00 |
|||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell autophagy | Activation | hsa04140 | |
| Cell apoptosis | Activation | hsa04210 | ||
| In Vitro Model | HSC3 cells | Tongue | Homo sapiens (Human) | CVCL_1288 |
| OVCAR3 cells | Ovary | Homo sapiens (Human) | CVCL_0465 | |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | There was statistical difference in the expression of the aforementioned proteins between the TFptreated group and TFptreated NC-LV group, but the autophagy level was upregulated and apoptosis was downregulated in the TFptreated NUPR1-LV group compared with the TFptreated NC-LV group. NUPR1 overexpression reversed the autophagic suppression and cellular apoptosis induction caused by TFP in U266 and RPMI 8226 cells. Thus, we concluded that TFP targeted NUPR1 in MM cells and subsequently induced apoptosis by inhibiting autophagy. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Bone marrow | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 3.83E-03; Fold-change: -4.02E-01; Z-score: -1.16E+00 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
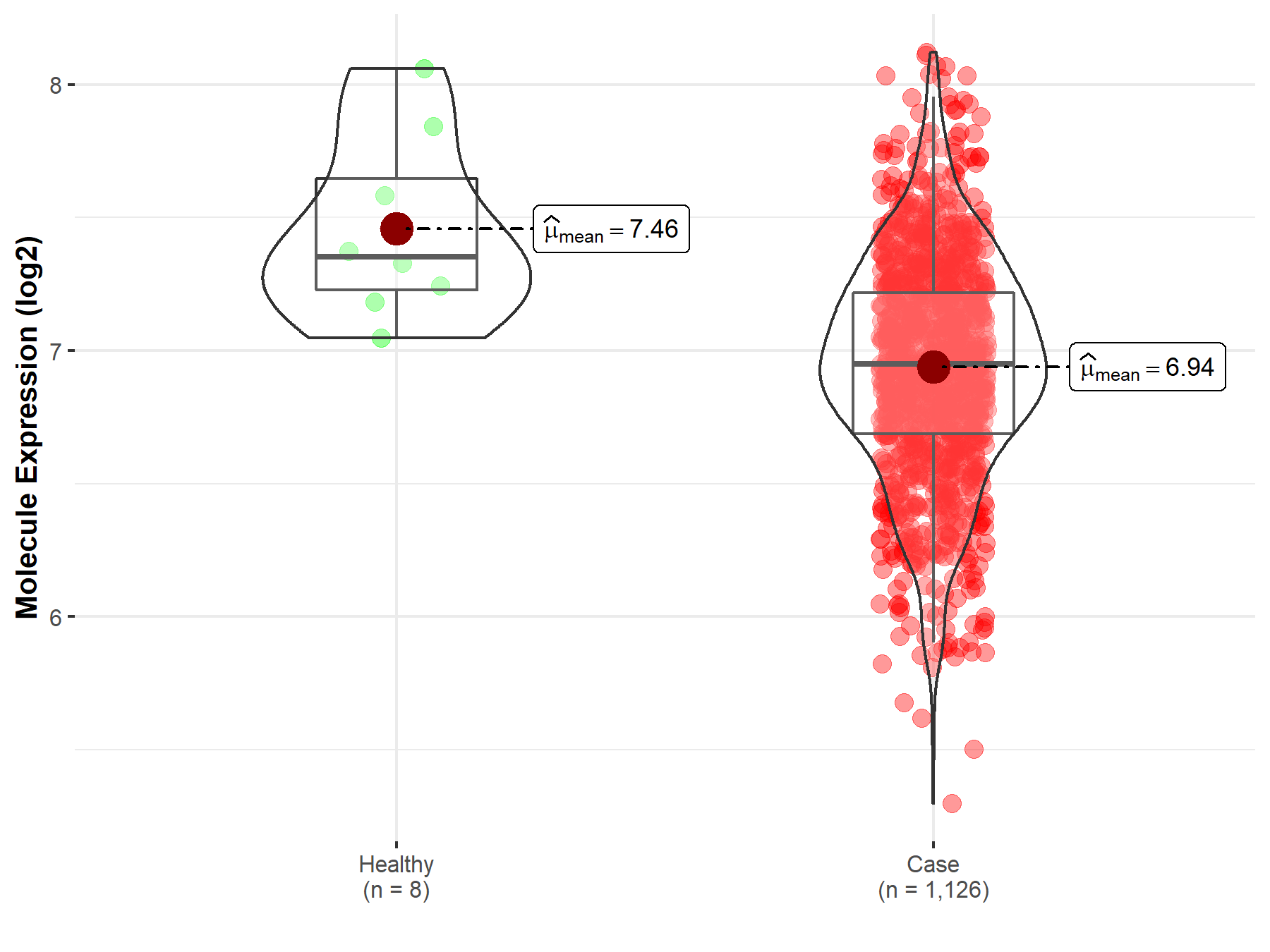
|
Click to View the Clearer Original Diagram |
| The Studied Tissue | Peripheral blood | |
| The Specified Disease | Multiple myeloma | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.19E-01; Fold-change: -1.24E-01; Z-score: -3.87E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
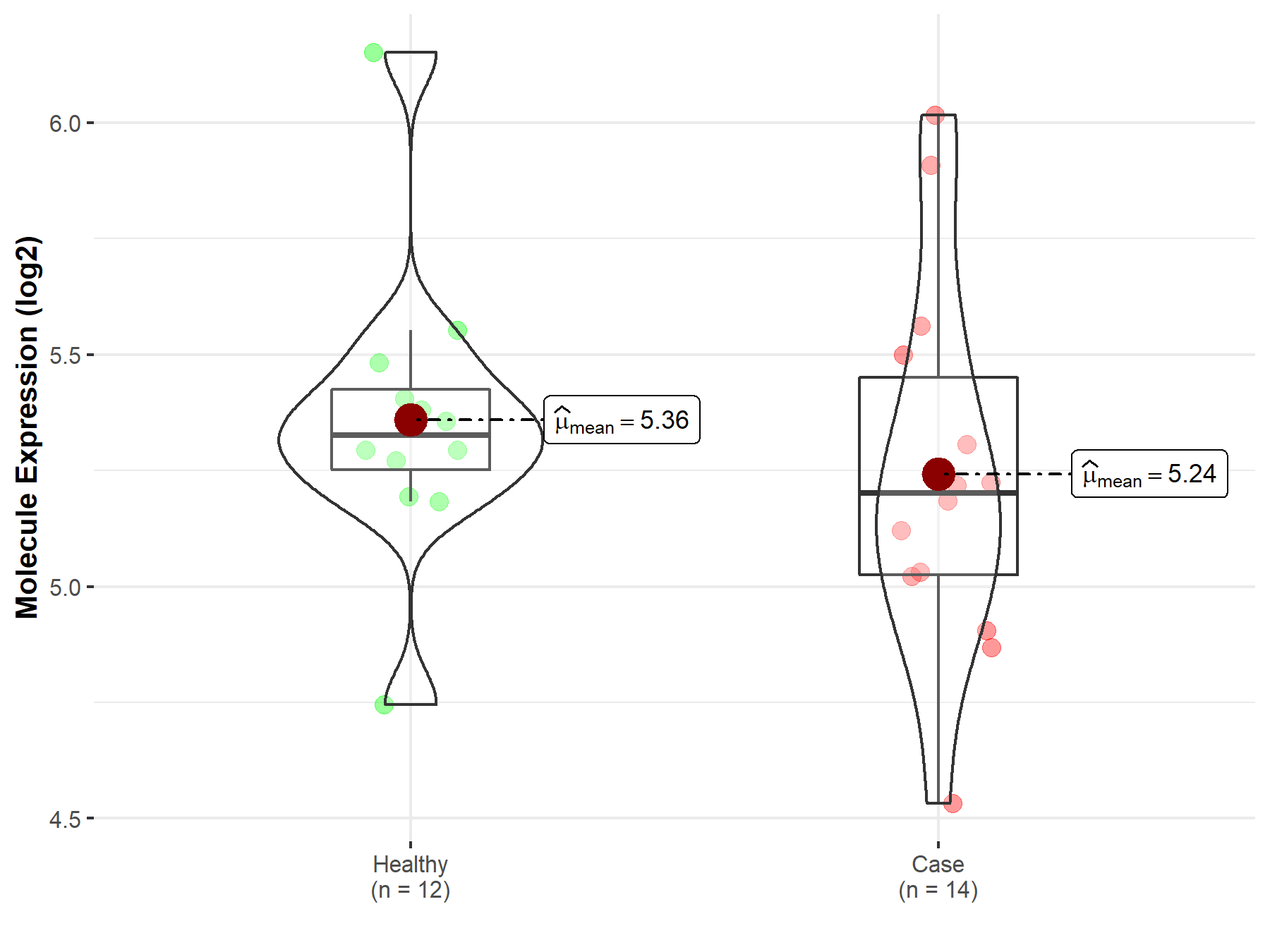
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

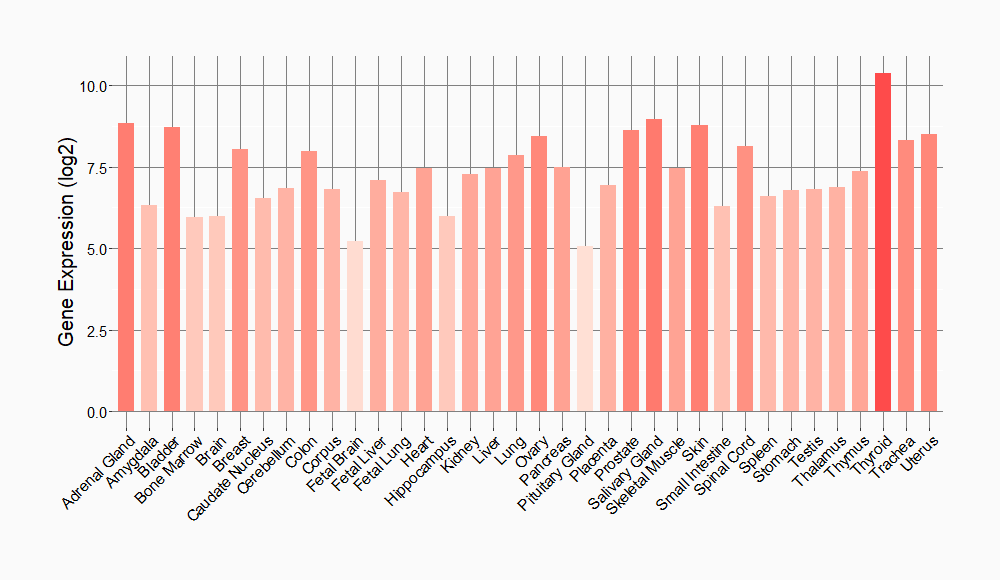
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
