Molecule Information
General Information of the Molecule (ID: Mol00376)
| Name |
Hepatocyte nuclear factor 3-alpha (FOXA1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Synonyms |
HNF-3-alpha; HNF-3A; Forkhead box protein A1; Transcription factor 3A; TCF-3A; HNF3A; TCF3A
Click to Show/Hide
|
||||
| Molecule Type |
Protein
|
||||
| Gene Name |
FOXA1
|
||||
| Gene ID | |||||
| Location |
chr14:37589552-37596059[-]
|
||||
| Sequence |
MLGTVKMEGHETSDWNSYYADTQEAYSSVPVSNMNSGLGSMNSMNTYMTMNTMTTSGNMT
PASFNMSYANPGLGAGLSPGAVAGMPGGSAGAMNSMTAAGVTAMGTALSPSGMGAMGAQQ AASMNGLGPYAAAMNPCMSPMAYAPSNLGRSRAGGGGDAKTFKRSYPHAKPPYSYISLIT MAIQQAPSKMLTLSEIYQWIMDLFPYYRQNQQRWQNSIRHSLSFNDCFVKVARSPDKPGK GSYWTLHPDSGNMFENGCYLRRQKRFKCEKQPGAGGGGGSGSGGSGAKGGPESRKDPSGA SNPSADSPLHRGVHGKTGQLEGAPAPGPAASPQTLDHSGATATGGASELKTPASSTAPPI SSGPGALASVPASHPAHGLAPHESQLHLKGDPHYSFNHPFSINNLMSSSEQQHKLDFKAY EQALQYSPYGSTLPASLPLGSASVTTRSPIEPSALEPAYYQGVYSRPVLNTS Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Transcription factor that is involved in embryonic development, establishment of tissue-specific gene expression and regulation of gene expression in differentiated tissues. Is thought to act as a 'pioneer' factor opening the compacted chromatin for other proteins through interactions with nucleosomal core histones and thereby replacing linker histones at target enhancer and/or promoter sites. Binds DNA with the consensus sequence 5'-[AC]A[AT]T[AG]TT[GT][AG][CT]T[CT]-3' (By similarity). Proposed to play a role in translating the epigenetic signatures into cell type-specific enhancer-driven transcriptional programs. Its differential recruitment to chromatin is dependent on distribution of histone H3 methylated at 'Lys-5' (H3K4me2) in estrogen-regulated genes. Involved in the development of multiple endoderm-derived organ systems such as liver, pancreas, lung and prostate; FOXA1 and FOXA2 seem to have at least in part redundant roles (By similarity). Modulates the transcriptional activity of nuclear hormone receptors. Is involved in ESR1-mediated transcription; required for ESR1 binding to the NKX2-1 promoter in breast cancer cells; binds to the RPRM promoter and is required for the estrogen-induced repression of RPRM. Involved in regulation of apoptosis by inhibiting the expression of BCL2. Involved in cell cycle regulation by activating expression of CDKN1B, alone or in conjunction with BRCA1. Originally described as a transcription activator for a number of liver genes such as AFP, albumin, tyrosine aminotransferase, PEPCK, etc. Interacts with the cis-acting regulatory regions of these genes. Involved in glucose homeostasis.
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
1 drug(s) in total
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | [1] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| In Vitro Model | CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 |
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
MTT assay | |||
| Mechanism Description | miR-132 can restore cisplatin treatment response in cisplatin-resistant xenografts in vivo, while FOXA1 protein levels were decreased. | |||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [2] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Revealed Based on the Cell Line Data | |||
| Cell Pathway Regulation | Cell apoptosis | Activation | hsa04210 | |
| Cell invasion | Inhibition | hsa05200 | ||
| Cell migration | Inhibition | hsa04670 | ||
| Cell proliferation | Inhibition | hsa05200 | ||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 |
| SPC-A1 cells | Lung | Homo sapiens (Human) | CVCL_6955 | |
| H1299 cells | Lung | Homo sapiens (Human) | CVCL_0060 | |
| Sk-MES-1 cells | Lung | Homo sapiens (Human) | CVCL_0630 | |
| 95D cells | Lung | Homo sapiens (Human) | CVCL_7110 | |
| 95C cells | Lung | Homo sapiens (Human) | CVCL_7109 | |
| Experiment for Molecule Alteration |
qRT-PCR; Western blot analysis | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Ectopic stable expression miR-194 suppressed proliferation, migration, invasion and metastasis and induced apoptosis in NSCLC cells and that this suppression could be reversed by reintroducing forkhead box A1 (FOXA1), a functional target of miR-194. In addition, miR-194 was downregulated in in cisplatin-resisted human NSCLC cell line-A549/DDP and overexpression of miR-194 increases cisplatin sensitivity. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.43E-59; Fold-change: 1.23E+00; Z-score: 2.23E+00 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 4.74E-37; Fold-change: 1.21E+00; Z-score: 1.98E+00 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |

|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

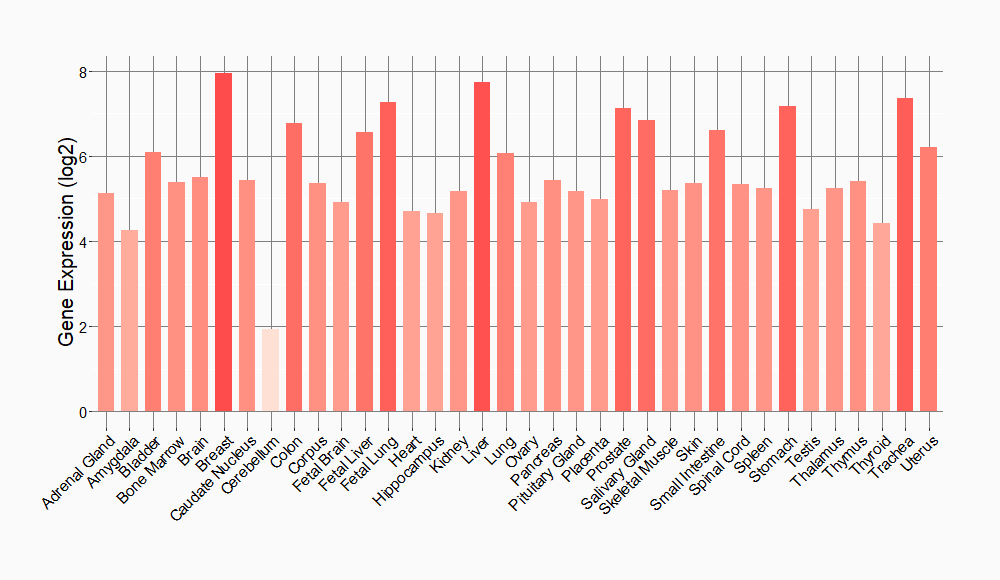
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
