Molecule Information
General Information of the Molecule (ID: Mol00121)
| Name |
Metastasis-associated protein MTA1 (MTA1)
,Homo sapiens
|
||||
|---|---|---|---|---|---|
| Molecule Type |
Protein
|
||||
| Gene Name |
MTA1
|
||||
| Gene ID | |||||
| Location |
chr14:105419820-105470729[+]
|
||||
| Sequence |
MAANMYRVGDYVYFENSSSNPYLIRRIEELNKTANGNVEAKVVCFYRRRDISSTLIALAD
KHATLSVCYKAGPGADNGEEGEIEEEMENPEMVDLPEKLKHQLRHRELFLSRQLESLPAT HIRGKCSVTLLNETESLKSYLEREDFFFYSLVYDPQQKTLLADKGEIRVGNRYQADITDL LKEGEEDGRDQSRLETQVWEAHNPLTDKQIDQFLVVARSVGTFARALDCSSSVRQPSLHM SAAAASRDITLFHAMDTLHKNIYDISKAISALVPQGGPVLCRDEMEEWSASEANLFEEAL EKYGKDFTDIQQDFLPWKSLTSIIEYYYMWKTTDRYVQQKRLKAAEAESKLKQVYIPNYN KPNPNQISVNNVKAGVVNGTGAPGQSPGAGRACESCYTTQSYQWYSWGPPNMQCRLCASC WTYWKKYGGLKMPTRLDGERPGPNRSNMSPHGLPARSSGSPKFAMKTRQAFYLHTTKLTR IARRLCREILRPWHAARHPYLPINSAAIKAECTARLPEASQSPLVLKQAVRKPLEAVLRY LETHPRPPKPDPVKSVSSVLSSLTPAKVAPVINNGSPTILGKRSYEQHNGVDGNMKKRLL MPSRGLANHGQARHMGPSRNLLLNGKSYPTKVRLIRGGSLPPVKRRRMNWIDAPDDVFYM ATEETRKIRKLLSSSETKRAARRPYKPIALRQSQALPPRPPPPAPVNDEPIVIED Click to Show/Hide
|
||||
| 3D-structure |
|
||||
| Function |
Transcriptional coregulator which can act as both a transcriptional corepressor and coactivator. As a part of the histone-deacetylase multiprotein complex (NuRD), regulates transcription of its targets by modifying the acetylation status of the target chromatin and cofactor accessibility to the target DNA. In conjunction with other components of NuRD, acts as a transcriptional corepressor of BRCA1, ESR1, TFF1 and CDKN1A. Acts as a transcriptional coactivator of BCAS3, PAX5 and SUMO2, independent of the NuRD complex. Stimulates the expression of WNT1 by inhibiting the expression of its transcriptional corepressor SIX3. Regulates p53-dependent and -independent DNA repair processes following genotoxic stress. Regulates the stability and function of p53/TP53 by inhibiting its ubiquitination by COP1 and MDM2 thereby regulating the p53-dependent DNA repair. Plays an important role in tumorigenesis, tumor invasion, and metastasis. Involved in the epigenetic regulation of ESR1 expression in breast cancer in a TFAP2C, IFI16 and HDAC4/5/6-dependent manner. Plays a role in the regulation of the circadian clock and is essential for the generation and maintenance of circadian rhythms under constant light and for normal entrainment of behavior to light-dark (LD) cycles. Positively regulates the CLOCK-ARNTL/BMAL1 heterodimer mediated transcriptional activation of its own transcription and the transcription of CRY1. Regulates deacetylation of ARNTL/BMAL1 by regulating SIRT1 expression, resulting in derepressing CRY1-mediated transcription repression. Isoform Short binds to ESR1 and sequesters it in the cytoplasm and enhances its non-genomic responses. With TFCP2L1, promotes establishment and maintenance of pluripotency in embryonic stem cells (ESCs) and inhibits endoderm differentiation (By similarity).
Click to Show/Hide
|
||||
| Uniprot ID | |||||
| Ensembl ID | |||||
| HGNC ID | |||||
| Click to Show/Hide the Complete Species Lineage | |||||
Type(s) of Resistant Mechanism of This Molecule
Drug Resistance Data Categorized by Drug
Approved Drug(s)
2 drug(s) in total
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Prostate cancer [ICD-11: 2C82.0] | [1] | |||
| Resistant Disease | Prostate cancer [ICD-11: 2C82.0] | |||
| Resistant Drug | Docetaxel | |||
| Molecule Alteration | Expression | Up-regulation |
||
| Differential expression of the molecule in resistant disease | ||||
| Classification of Disease | Prostate cancer [ICD-11: 2C82] | |||
| The Specified Disease | Prostate cancer | |||
| The Studied Tissue | Prostate | |||
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.16E-01 Fold-change: 4.86E-02 Z-score: 1.64E+00 |
|||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | MTA1 signaling pathway | Activation | hsa05206 | |
| In Vitro Model | LNCaP cells | Prostate | Homo sapiens (Human) | CVCL_0395 |
| PC3 cells | Prostate | Homo sapiens (Human) | CVCL_0035 | |
| Experiment for Molecule Alteration |
Promoter reporter assay; Western blot analysis | |||
| Experiment for Drug Resistance |
ELISA; MTT assay | |||
| Mechanism Description | Regulation of docetaxel sensitivity in prostate cancer cells by hsa-miR125a-3p via modulation of metastasis-associated protein 1 signaling, MTA1 is a direct target of hsa-mir125a-3p in pca cells. | |||
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Disease Class: Non-small cell lung cancer [ICD-11: 2C25.Y] | [2] | |||
| Sensitive Disease | Non-small cell lung cancer [ICD-11: 2C25.Y] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell invasion | Inhibition | hsa05200 | |
| In Vitro Model | TL-1 cells | Lung | Homo sapiens (Human) | CVCL_B371 |
| Experiment for Molecule Alteration |
RT-PCR | |||
| Experiment for Drug Resistance |
Immunohistochemical staining assay | |||
| Mechanism Description | miR-30c-2* negative regulated MTA-1 expression involved in metastasis and reducing drug resistance of HPV-infected non-small cell lung cancer. | |||
|
|
||||
| Disease Class: Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | [3] | |||
| Sensitive Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B.0] | |||
| Sensitive Drug | Cisplatin | |||
| Molecule Alteration | Expression | Down-regulation |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| Cell Pathway Regulation | Cell cytotoxicity | Activation | hsa04650 | |
| Tumorigenesis | Inhibition | hsa05200 | ||
| In Vitro Model | 5-8F cells | Nasopharynx | Homo sapiens (Human) | CVCL_C528 |
| CNE2 cells | Nasopharynx | Homo sapiens (Human) | CVCL_6889 | |
| C666-1 cells | Throat | Homo sapiens (Human) | CVCL_7949 | |
| CNE1 cells | Throat | Homo sapiens (Human) | CVCL_6888 | |
| HONE1 cells | Throat | Homo sapiens (Human) | CVCL_8706 | |
| In Vivo Model | Nude mouse xenograft model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis; Luciferase reporter assay | |||
| Experiment for Drug Resistance |
MTT assay; Flow cytometric analysis | |||
| Mechanism Description | miR183 overexpression inhibits tumorigenesis and enhances DDP-induced cytotoxicity by targeting MTA1 in nasopharyngeal carcinoma. | |||
Disease- and Tissue-specific Abundances of This Molecule
ICD Disease Classification 02

| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Lung | |
| The Specified Disease | Lung cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 4.98E-14; Fold-change: 2.99E-01; Z-score: 6.66E-01 | |
| The Expression Level of Disease Section Compare with the Adjacent Tissue | p-value: 2.58E-01; Fold-change: -8.59E-02; Z-score: -2.07E-01 | |
|
Molecule expression in the normal tissue adjacent to the diseased tissue of patients
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
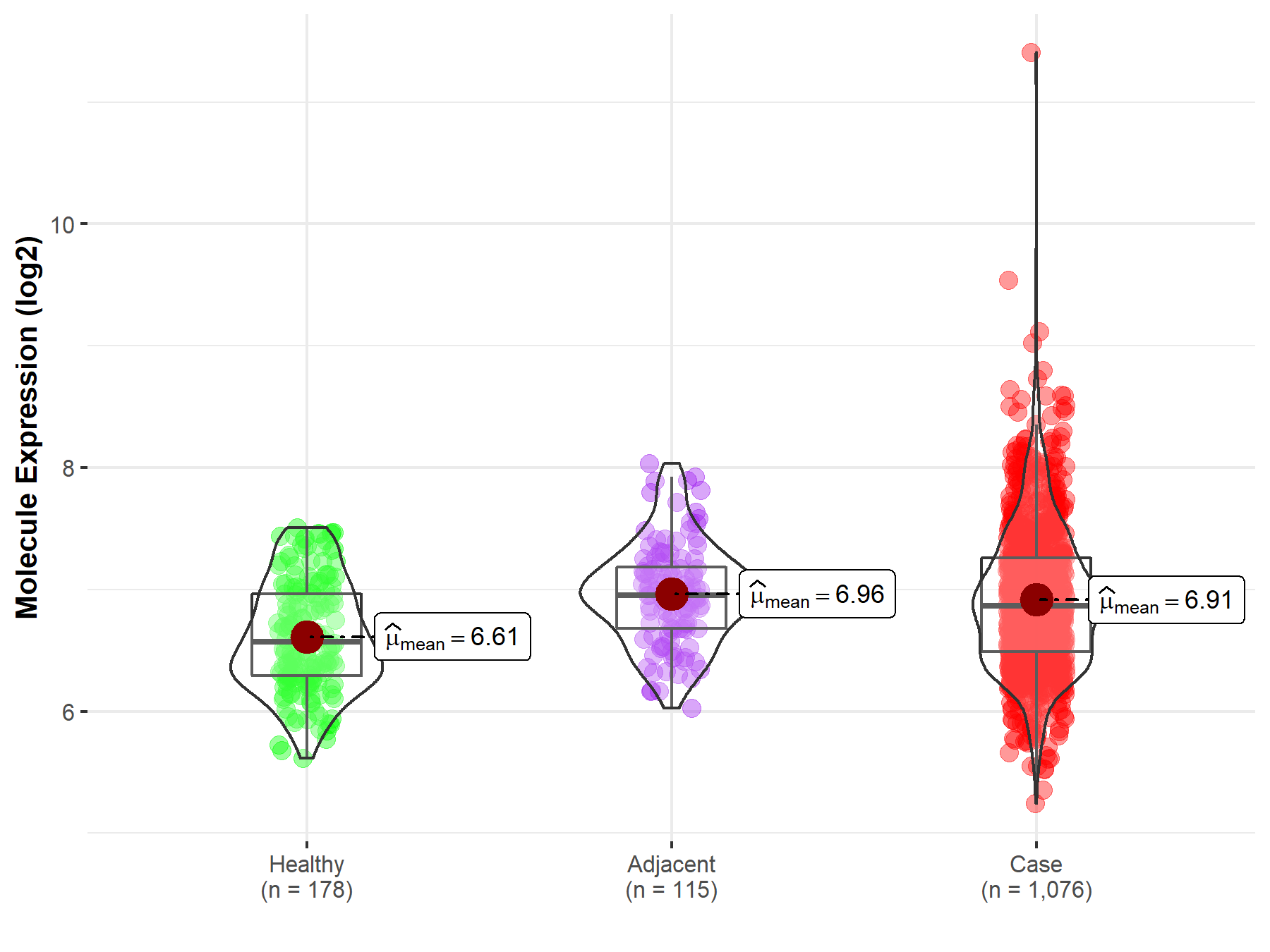
|
Click to View the Clearer Original Diagram |
| Differential expression of molecule in resistant diseases | ||
| The Studied Tissue | Prostate | |
| The Specified Disease | Prostate cancer | |
| The Expression Level of Disease Section Compare with the Healthy Individual Tissue | p-value: 1.16E-01; Fold-change: 2.75E-01; Z-score: 3.89E-01 | |
|
Molecule expression in the diseased tissue of patients
Molecule expression in the normal tissue of healthy individuals
|
||
| Disease-specific Molecule Abundances |
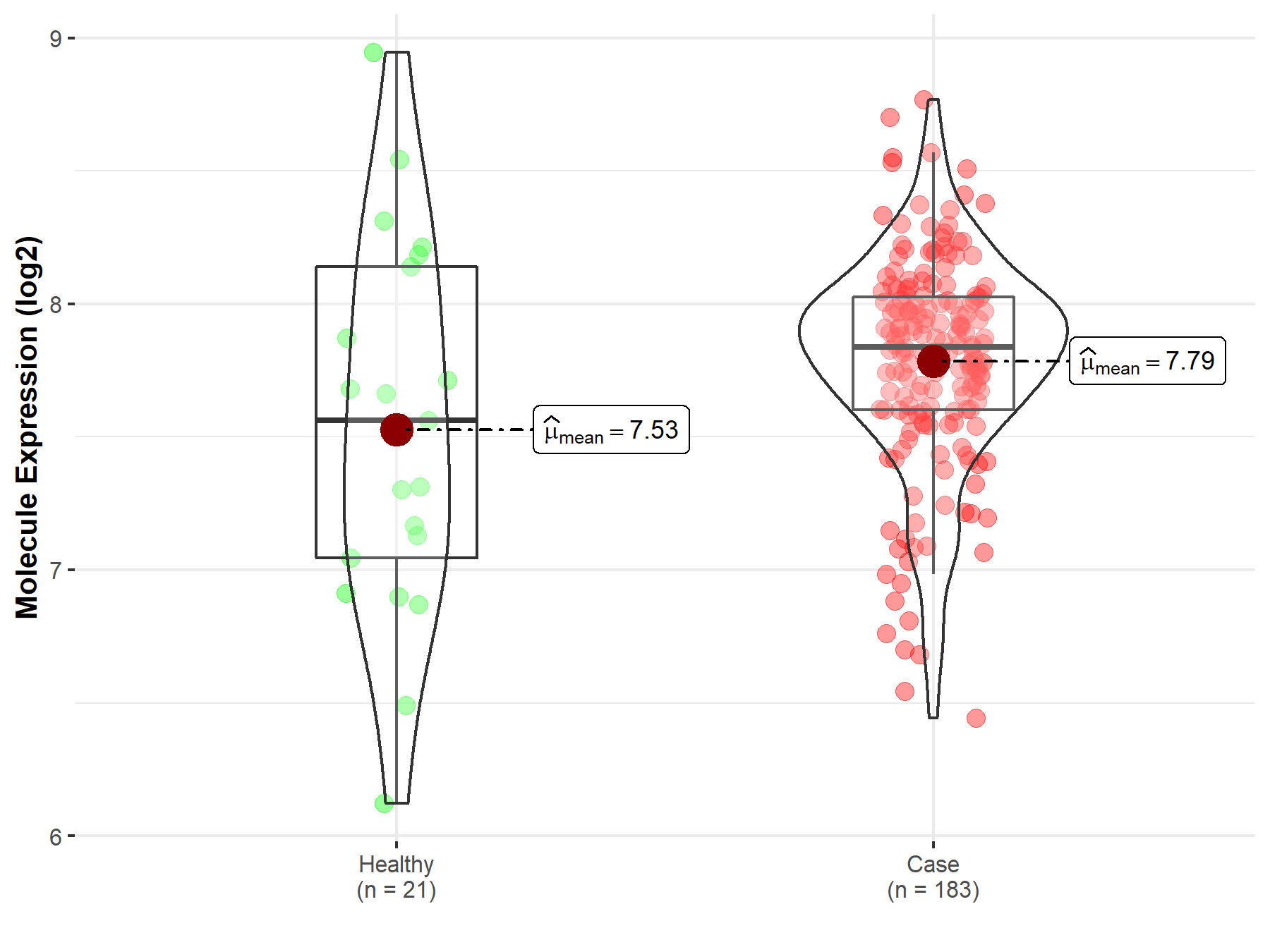
|
Click to View the Clearer Original Diagram |
Tissue-specific Molecule Abundances in Healthy Individuals

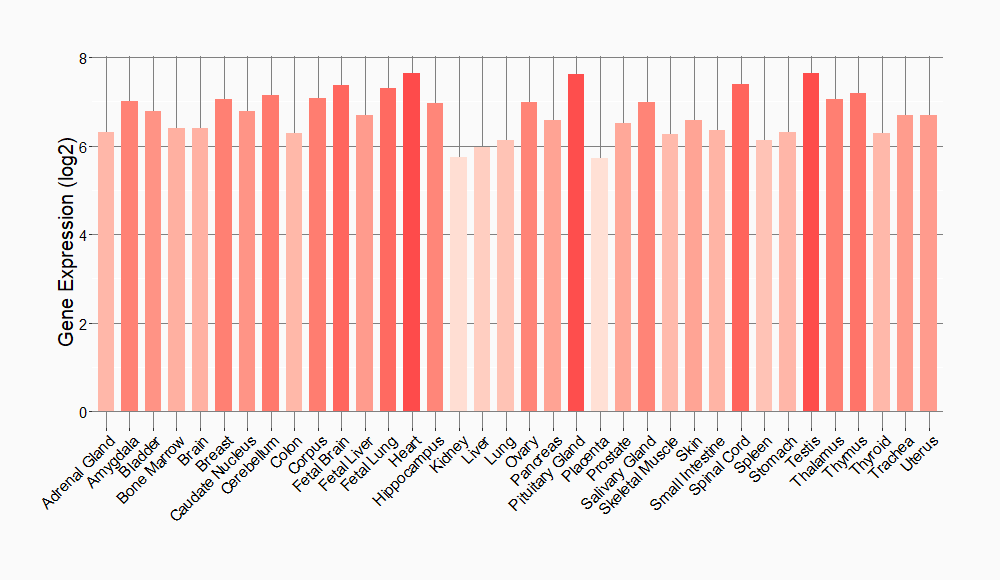
|
||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
