Drug Information
Drug (ID: DG01773) and It's Reported Resistant Information
| Name |
Hydroquinone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Hydroquinone; Benzene-1,4-diol; 1,4-benzenediol; 123-31-9; Quinol; 1,4-Dihydroxybenzene; p-Benzenediol; p-Hydroquinone; p-Hydroxyphenol; p-Dihydroxybenzene; 4-Hydroxyphenol; Benzoquinol; Eldoquin; hydroquinol; Eldopaque; Phiaquin; p-Dioxybenzene; Dihydroquinone; Hydroquinole; Idrochinone; Tecquinol; Dihydroxybenzene; Solaquin forte; Benzohydroquinone; Arctuvin; Hidroquinone; Tequinol; Derma-Blanch; Tenox HQ; Hydrochinon; Hydrochinone; Artra; Eldopaque Forte; Eldoquin Forte; Diak 5; Benzene, p-dihydroxy-; 1,4-Dihydroxy-benzol; Usaf ek-356; 1,4-Diidrobenzene; p-Dioxobenzene; 1,4-Dihydroxybenzen; para-Dioxybenzene; para-Hydroquinone; Pyrogentistic acid; 1,4-Dihydroxy-benzeen; NCI-C55834; HE 5; para-Dihydroxybenzene; Black and White Bleaching Cream; Melanex; Idrochinone [Italian]; Hydrochinon [Czech, Polish]; 1,4-Dihydroxybenzen [Czech]; 1,4-Diidrobenzene [Italian]; 1,4-Dihydroxy-benzeen [Dutch]; 1,4-Dihydroxy-benzol [German]; UNII-XV74C1N1AE; NSC 9247; UN2662; Hydroquinone [USP]; CHEBI:17594; AI3-00072; 4-DIHYDROXYBENZENE; MFCD00002339; HQ; CHEMBL537; XV74C1N1AE; 1,4-Benzenediol, homopolymer; DTXSID7020716; NSC-9247; Hydroquinone [UN2662] [Poison]; Hydroquinone (USP); NCGC00015523-02; beta-quinol; DSSTox_CID_716; para-Hydroxyphenol; DSSTox_RID_75754; DSSTox_GSID_20716; Eldopacque; Hydroquinone (Benzene-1,4-diol); Epiquin; Sunvanish; p Benzendiol; p-Dihydroquinone; alpha-hydroquinone; 26982-52-5; CAS-123-31-9; SMR000059154; CCRIS 714; 1,4-Hydroxybenzene; HSDB 577; SR-01000075920; EINECS 204-617-8; hydroquinon; Hydroquinoue; hydroq uinone; hydroquinone gr; a-Hydroquinone; Black & White Bleaching Cream; p-Hydroxybenzene; b-Quinol; 4-Benzenediol; Hydroquinone, HQ; .beta.-Quinol; 1,4 benzenediol; Hydroquinone,(S); p-dihydroxy benzene; HQE; Hydroquinone polymer; PLQ; Artra (Salt/Mix); 1, 4-Benzenediol; HYDROP; .alpha.-Hydroquinone; phenol derivative, 4; 4-hydroxyphenyl alcohol; Spectrum_001757; 4e3h; SpecPlus_000769; 1,4-Dihydrobenzoquinone; ELDOQUIN (TN); hydroquinone for synthesis; Spectrum2_001672; Spectrum3_000656; Spectrum4_000633; Spectrum5_001430; Lopac-H-9003; 1,2 BENZOLDIOL; WLN: QR DQ; bmse000293; Epitope ID:116206; EC 204-617-8; Lopac0_000577; SCHEMBL15516; BSPBio_002291; KBioGR_001246; KBioSS_002237; 1,4-Dihydroxybenzene, XIII; Hydroquinone-1,4-Benzenediol; MLS000069815; MLS001074911; BIDD:ER0340; DivK1c_006865; Hydroquinone, LR, >=99%; SPECTRUM1504237; Hydrochinon(CZECH, POLISH); SPBio_001883; BDBM26190; Hydroquinone, puriss., 99.0%; KBio1_001809; KBio2_002237; KBio2_004805; KBio2_007373; KBio3_001511; NSC9247; Benzene-1,4-diol (Hydroquinone); HMS1922H15; HMS2093E08; HMS3261D16; LABOTEST-BB LTBB001931; Pharmakon1600-01504237; HY-B0951; ZINC5133378; Tox21_110169; Tox21_202345; Tox21_300015; Tox21_500577; CCG-39082; NSC758707; s4580; STK397446; AKOS000119003; Tox21_110169_1; AM10548; AS00174; DB09526; LP00577; MCULE-3953269041; NSC-758707; SDCCGSBI-0050559.P003; UN 2662; Hydroquinone, ReagentPlus(R), >=99%; Hydroquinone, USP, 99.0-100.5%; NCGC00015523-01; NCGC00015523-03; NCGC00015523-04; NCGC00015523-05; NCGC00015523-06; NCGC00015523-07; NCGC00015523-08; NCGC00015523-09; NCGC00015523-10; NCGC00015523-11; NCGC00015523-12; NCGC00015523-13; NCGC00015523-19; NCGC00090880-01; NCGC00090880-02; NCGC00090880-03; NCGC00090880-04; NCGC00090880-05; NCGC00254037-01; NCGC00259894-01; NCGC00261262-01; BP-21160; DA-33570; Hydroquinone, ReagentPlus(R), >=99.5%; SBI-0050559.P002; Hydroquinone, SAJ first grade, >=99.0%; EU-0100577; FT-0606877; H0186; Hydroquinone, SAJ special grade, >=99.0%; Hydroquinone, meets USP testing specifications; C00530; D00073; H 9003; AB00053361_08; Quinol; 1,4-Benzenediol; 1,4-Dihydroxybenzene; Q419164; J-004910; J-521469; SR-01000075920-1; SR-01000075920-4; Q27102742; Z57127551; 094CADDB-59BF-4EDF-B278-59791B203EA2; F1908-0167; Hydroquinone, certified reference material, TraceCERT(R); Hydroquinone, United States Pharmacopeia (USP) Reference Standard; Hydroquinone, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Indication |
In total 1 Indication(s)
|
||||
| Structure |
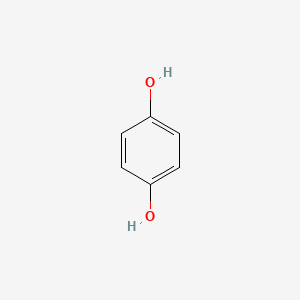
|
||||
| Drug Resistance Disease(s) |
Disease(s) with Clinically Reported Resistance for This Drug
(1 diseases)
[1]
|
||||
| Target | Glycoprotein IIb/IIIa receptor (GPIIb/IIIa) |
ITA2B_HUMAN
; ITB3_HUMAN |
[1] | ||
| Tyrosinase (TYR) | TYRO_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| IsoSMILES |
C1=CC(=CC=C1O)O
|
||||
| InChI |
InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H
|
||||
| InChIKey |
QIGBRXMKCJKVMJ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| ChEBI ID | |||||
| TTD Drug ID | |||||
| VARIDT ID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Resistance Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: FAS antisense RNA 1 (FAS-AS1) | [1] | |||
| Resistant Disease | Acute lymphocytic leukemia [ICD-11: 2B33.0] | |||
| Molecule Alteration | Down-regulation | Expression |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | TK6 cells | Spleen | Homo sapiens (Human) | CVCL_0561 |
| In Vivo Model | BALB/c nude mice model | Mus musculus | ||
| Experiment for Molecule Alteration |
qRT-PCR; Knockdown assay; Overexpression assay | |||
| Experiment for Drug Resistance |
CCK8 assay | |||
| Mechanism Description | Aken together, these observations demonstrate crosstalk between FAS-AS1 and DNMT3b via a mutual inhibition loop and indicate a new mechanism by which FAS-AS1 regulates the expression of FAS in benzene-related carcinogenesis. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
