Drug Information
Drug (ID: DG01664) and It's Reported Resistant Information
| Name |
Cixutumumab
|
||||
|---|---|---|---|---|---|
| Synonyms |
SCHEMBL20511307
Click to Show/Hide
|
||||
| Indication |
In total 2 Indication(s)
|
||||
| Structure |
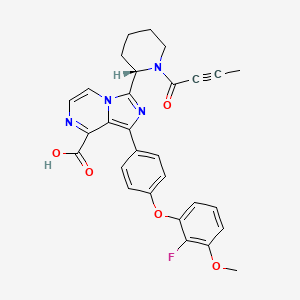
|
||||
| Target | Insulin-like growth factor I receptor (IGF1R) | IGF1R_HUMAN | [1] | ||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
6
|
||||
| IsoSMILES |
CC#CC(=O)N1CCCC[C@H]1C2=NC(=C3N2C=CN=C3C(=O)O)C4=CC=C(C=C4)OC5=CC=CC(=C5F)OC
|
||||
| InChI |
InChI=1S/C29H25FN4O5/c1-3-7-23(35)33-16-5-4-8-20(33)28-32-25(27-26(29(36)37)31-15-17-34(27)28)18-11-13-19(14-12-18)39-22-10-6-9-21(38-2)24(22)30/h6,9-15,17,20H,4-5,8,16H2,1-2H3,(H,36,37)/t20-/m0/s1
|
||||
| InChIKey |
BPGVVRUPKNFLHW-FQEVSTJZSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Protein-tyrosine phosphatase delta (PTPRD) | [1] | |||
| Sensitive Disease | Ewing sarcoma [ICD-11: 2B52.0] | |||
| Molecule Alteration | Missense mutation | p.V253I (c.757G>A) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Ewing sarcoma tissue | N.A. | ||
| Experiment for Molecule Alteration |
DNA sequencing assay | |||
| Mechanism Description | The missense mutation p.V253I (c.757G>A) in gene PTPRD cause the sensitivity of Cixutumumab by unusual activation of pro-survival pathway | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
