Drug Information
Drug (ID: DG01639) and It's Reported Resistant Information
| Name |
MRX-2843
|
||||
|---|---|---|---|---|---|
| Synonyms |
MRX-2843; 1429882-07-4; UNC-2371A; UNII-2MT30EHI63; 2MT30EHI63; CHEMBL3326007; (1r,4r)-4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol; Cyclohexanol, 4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methyl-1-piperazinyl)methyl)phenyl)-7H-pyrrolo(2,3-d)pyrimidin-7-yl)-, trans-; trans-4-(2-((2-Cyclopropylethyl)amino)-5-(4-((4-methyl-1-piperazinyl)methyl)phenyl)-7H-pyrrolo(2,3-d)pyrimidin-7-yl)cyclohexanol; UNC2371A; SCHEMBL14854108; SCHEMBL17175579; UNC2371UNC2371; BDBM350861; BCP30180; BDBM50055490; MFCD28502224; s6839; ZINC299829706; CS-8117; SB17283; AS-35252; HY-101549; MRX-2843; (UNC-2371A); US9795606, B20; A16958; UNC2371;UNC-2371;UNC 2371;MRX 2843 ;MRX2843; 4-[2-(2-cyclopropylethylamino)-5-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]pyrrolo[2,3-d]pyrimidin-7-yl]cyclohexan-1-ol; trans-(1R,4R)-4-(2-((2-cyclopropylethyl)amino)-5-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)cyclohexanol
Click to Show/Hide
|
||||
| Indication |
In total 3 Indication(s)
|
||||
| Structure |
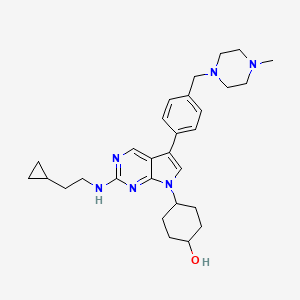
|
||||
| Target | Fibroblast growth factor receptor 1 (FGFR1) | FGFR1_HUMAN | [1] | ||
| Fibroblast growth factor receptor 2 (FGFR2) | FGFR2_HUMAN | [1] | |||
| Fibroblast growth factor receptor 3 (FGFR3) | FGFR3_HUMAN | [1] | |||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
8
|
||||
| IsoSMILES |
CN1CCN(CC1)CC2=CC=C(C=C2)C3=CN(C4=NC(=NC=C34)NCCC5CC5)C6CCC(CC6)O
|
||||
| InChI |
InChI=1S/C29H40N6O/c1-33-14-16-34(17-15-33)19-22-4-6-23(7-5-22)27-20-35(24-8-10-25(36)11-9-24)28-26(27)18-31-29(32-28)30-13-12-21-2-3-21/h4-7,18,20-21,24-25,36H,2-3,8-17,19H2,1H3,(H,30,31,32)
|
||||
| InChIKey |
LBEJYFVJIPQSNX-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | ||||
|
|
||||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D835V (c.2504A>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 |
| MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| NOMO-1 cells | Bone marrow | Homo sapiens (Human) | CVCL_1609 | |
| In Vivo Model | NSG mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
FACS assay | |||
| Mechanism Description | MRX-2843, a type 1 small-molecule tyrosine kinase inhibitor that abrogates activation of both MERTK and FLT3 and their downstream effectors. MRX-2843 treatment induces apoptosis and inhibits colony formation in AML cell lines and primary patient samples expressing MERTK and/or FLT3-ITD, with a wide therapeutic window compared with that of normal human cord blood cells. | |||
| Key Molecule: Receptor-type tyrosine-protein kinase FLT3 (FLT3) | [1] | |||
| Sensitive Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
| Molecule Alteration | Missense mutation | p.D835Y (c.2503G>T) |
||
| Experimental Note | Identified from the Human Clinical Data | |||
| In Vitro Model | Kasumi-1 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0589 |
| MOLM-14 cells | Peripheral blood | Homo sapiens (Human) | CVCL_7916 | |
| MV4-11 cells | Peripheral blood | Homo sapiens (Human) | CVCL_0064 | |
| NOMO-1 cells | Bone marrow | Homo sapiens (Human) | CVCL_1609 | |
| In Vivo Model | NSG mouse PDX model | Mus musculus | ||
| Experiment for Molecule Alteration |
Western blot analysis | |||
| Experiment for Drug Resistance |
FACS assay | |||
| Mechanism Description | MRX-2843, a type 1 small-molecule tyrosine kinase inhibitor that abrogates activation of both MERTK and FLT3 and their downstream effectors. MRX-2843 treatment induces apoptosis and inhibits colony formation in AML cell lines and primary patient samples expressing MERTK and/or FLT3-ITD, with a wide therapeutic window compared with that of normal human cord blood cells. | |||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
