Drug Information
Drug (ID: DG01634) and It's Reported Resistant Information
| Name |
AZD-3759
|
||||
|---|---|---|---|---|---|
| Synonyms |
AZD3759; 1626387-80-1; zorifertinib; AZD-3759; AZD 3759; (R)-4-((3-chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl 2,4-dimethylpiperazine-1-carboxylate; UNII-67SX9H68W2; CHEMBL3623290; 67SX9H68W2; 4-[(3-CHLORO-2-FLUOROPHENYL)AMINO]-7-METHOXYQUINAZOLIN-6-YL (2R)-2,4-DIMETHYLPIPERAZINE-1-CARBOXYLATE; (2R)-2,4-dimethyl-1-piperazinecarboxylic acid, 4-[(3-chloro-2-fluorophenyl)amino]-7-methoxy-6-quinazolinyl ester; [4-(3-chloro-2-fluoroanilino)-7-methoxyquinazolin-6-yl] (2R)-2,4-dimethylpiperazine-1-carboxylate; 4-((3-Chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl (2R)-2,4-dimethylpiperazine-1-carboxylate; SCHEMBL16010006; GTPL10456; EX-A720; AMY10302; BDBM50123453; MFCD29058564; NSC788121; NSC800978; s7971; AKOS027327321; ZINC221149242; CCG-269329; CS-5029; DB14795; NSC-788121; NSC-800978; compound 1m [PMID: 26313252]; NCGC00483922-01; NCGC00483922-02; AC-29957; AS-74755; HY-18750; A882818; (2R)-2,4-Dimethyl-1-piperazinecarboxylic acid 4-[(3-chloro-2-fluorophenyl)amino]-7-methoxy-6-quinazolinyl ester; (2R)-2,4-Dimethylpiperazine-1-carboxylic acid (4-(2-fluoro-3-chloroanilino)-7-methoxyquinazoline-6-yl) ester; (R)-4-(3-chloro-2-fluorophenylamino)-7-methoxyquinazolin-6-yl 2,4-dimethylpiperazine-1-carboxylate
Click to Show/Hide
|
||||
| Structure |
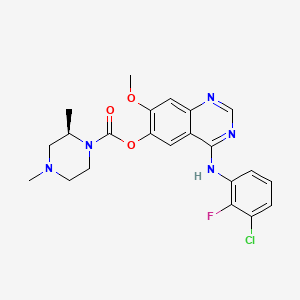
|
||||
| Click to Show/Hide the Molecular Information and External Link(s) of This Drug | |||||
| Formula |
5
|
||||
| IsoSMILES |
C[C@@H]1CN(CCN1C(=O)OC2=C(C=C3C(=C2)C(=NC=N3)NC4=C(C(=CC=C4)Cl)F)OC)C
|
||||
| InChI |
InChI=1S/C22H23ClFN5O3/c1-13-11-28(2)7-8-29(13)22(30)32-19-9-14-17(10-18(19)31-3)25-12-26-21(14)27-16-6-4-5-15(23)20(16)24/h4-6,9-10,12-13H,7-8,11H2,1-3H3,(H,25,26,27)/t13-/m1/s1
|
||||
| InChIKey |
MXDSJQHFFDGFDK-CYBMUJFWSA-N
|
||||
| PubChem CID | |||||
| DrugBank ID | |||||
Type(s) of Resistant Mechanism of This Drug
Drug Resistance Data Categorized by Their Corresponding Diseases
ICD-02: Benign/in-situ/malignant neoplasm
| Drug Sensitivity Data Categorized by Their Corresponding Mechanisms | |||||||||||||
|
|
|||||||||||||
| Key Molecule: Epidermal growth factor receptor (EGFR) | [1] | ||||||||||||
| Sensitive Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | ||||||||||||
| Molecule Alteration | Missense mutation | p.L858R (c.2573T>G) |
|||||||||||
| Wild Type Structure | Method: X-ray diffraction | Resolution: 2.64 Å | |||||||||||
| Mutant Type Structure | Method: X-ray diffraction | Resolution: 2.47 Å | |||||||||||
| Download The Information of Sequence | Download The Structure File | ||||||||||||
-
G
-
S
-
M
-
G
G
E
E
A
A
P
P
700
|
N
N
Q
Q
A
A
L
L
L
L
R
R
I
I
L
L
K
K
E
E
710
|
T
T
E
E
F
F
K
K
K
K
I
I
K
K
V
V
L
L
G
G
720
|
S
S
G
G
A
A
F
F
G
G
T
T
V
V
Y
Y
K
K
G
G
730
|
L
L
W
W
I
I
P
P
E
E
G
G
E
E
K
K
V
V
K
K
740
|
I
I
P
P
V
V
A
A
I
I
K
K
E
E
L
L
R
R
E
E
750
|
A
A
T
T
S
S
P
P
K
K
A
A
N
N
K
K
E
E
I
I
760
|
L
L
D
D
E
E
A
A
Y
Y
V
V
M
M
A
A
S
S
V
V
770
|
D
D
N
N
P
P
H
H
V
V
C
C
R
R
L
L
L
L
G
G
780
|
I
I
C
C
L
L
T
T
S
S
T
T
V
V
Q
Q
L
L
I
I
790
|
T
T
Q
Q
L
L
M
M
P
P
F
F
G
G
C
C
L
L
L
L
800
|
D
D
Y
Y
V
V
R
R
E
E
H
H
K
K
D
D
N
N
I
I
810
|
G
G
S
S
Q
Q
Y
Y
L
L
L
L
N
N
W
W
C
C
V
V
820
|
Q
Q
I
I
A
A
K
K
G
G
M
M
N
N
Y
Y
L
L
E
E
830
|
D
D
R
R
R
R
L
L
V
V
H
H
R
R
D
D
L
L
A
A
840
|
A
A
R
R
N
N
V
V
L
L
V
V
K
K
T
T
P
P
Q
Q
850
|
H
H
V
V
K
K
I
I
T
T
D
D
F
F
G
G
L
R
A
A
860
|
K
K
L
L
L
L
G
G
A
A
E
E
E
E
K
K
E
E
Y
Y
870
|
H
H
A
A
E
E
G
G
G
G
K
K
V
V
P
P
I
I
K
K
880
|
W
W
M
M
A
A
L
L
E
E
S
S
I
I
L
L
H
H
R
R
890
|
I
I
Y
Y
T
T
H
H
Q
Q
S
S
D
D
V
V
W
W
S
S
900
|
Y
Y
G
G
V
V
T
T
V
V
W
W
E
E
L
L
M
M
T
T
910
|
F
F
G
G
S
S
K
K
P
P
Y
Y
D
D
G
G
I
I
P
P
920
|
A
A
S
S
E
E
I
I
S
S
S
S
I
I
L
L
E
E
K
K
930
|
G
G
E
E
R
R
L
L
P
P
Q
Q
P
P
P
P
I
I
C
C
940
|
T
T
I
I
D
D
V
V
Y
Y
M
M
I
I
M
M
V
V
K
K
950
|
C
C
W
W
M
M
I
I
D
D
A
A
D
D
S
S
R
R
P
P
960
|
K
K
F
F
R
R
E
E
L
L
I
I
I
I
E
E
F
F
S
S
970
|
K
K
M
M
A
A
R
R
D
D
P
P
Q
Q
R
R
Y
Y
L
L
980
|
V
V
I
I
Q
Q
G
G
D
D
E
E
R
R
M
M
H
H
L
L
990
|
P
P
S
S
P
P
T
T
D
D
S
S
N
N
F
F
Y
Y
R
R
1000
|
A
A
L
L
M
M
D
D
E
E
E
E
D
D
M
M
D
D
D
D
1010
|
V
V
V
V
D
D
A
A
D
D
E
E
Y
Y
L
L
I
I
P
P
1020
|
Q
Q
-
Q
-
G
|
|||||||||||||
| Experimental Note | Identified from the Human Clinical Data | ||||||||||||
| Cell Pathway Regulation | EGFR signaling pathway | Inhibition | hsa01521 | ||||||||||
| In Vitro Model | A549 cells | Lung | Homo sapiens (Human) | CVCL_0023 | |||||||||
| HCC827 cells | Lung | Homo sapiens (Human) | CVCL_2063 | ||||||||||
| NCI-H838 cells | Lung | Homo sapiens (Human) | CVCL_1594 | ||||||||||
| NCI-H1975 cells | Lung | Homo sapiens (Human) | CVCL_1511 | ||||||||||
| PC9 cells | Lung | Homo sapiens (Human) | CVCL_B260 | ||||||||||
| NCI-H3255 cells | Lung | Homo sapiens (Human) | CVCL_6831 | ||||||||||
| In Vivo Model | Nude mouse PDX model | Mus musculus | |||||||||||
| Experiment for Drug Resistance |
Enzyme-linked immunosorbent assay | ||||||||||||
| Mechanism Description | AZD3759, a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. Treatment with AZD3759 causes tumor regression in subcutaneous xenograft, leptomeningeal metastasis (LM), and brain metastasis (BM) lung cancer models and prevents the development of BM in nude mice. | ||||||||||||
References
If you find any error in data or bug in web service, please kindly report it to Dr. Sun and Dr. Yu.
